Facial perception of conspecifics: chimpanzees (Pan troglodytes) attend to proper orientation and open eyes.
Satoshi Hirata, Koki Fuwa, Keiko Sugama, Kiyo Kusunoki, Shin Fujita
DOI: 10.1007/s10071-010-0316-yAbstract
This paper reports on the use of an eye-tracking technique to examine how chimpanzees look at facial photographs of conspeciWcs. Six chimpanzees viewed a sequence of pictures presented on a monitor while their eye movements were measured by an eye tracker. The pictures presented conspeciWc faces with open or closed eyes in an upright or inverted orientation in a frame. The results demonstrated that chimpanzees looked at the eyes, nose, and mouth more frequently than would be expected on the basis of random scanning of faces. More speciWcally, they looked at the eyes longer than they looked at the nose and mouth when photographs of upright faces with open eyes were presented, suggesting that particular attention to the eyes represents a spontaneous face-scanning strategy shared among monkeys, apes, and humans. In contrast to the results obtained for upright faces with open eyes, the viewing times for the eyes, nose, and mouth of inverted faces with open eyes did not diVer from one another. The viewing times for the eyes, nose, and mouth of faces with closed eyes did not diVer when faces with closed eyes were presented in either an upright or inverted orientation. These results suggest the possibility that open eyes play an important role in the conWgural processing of faces and that chimpanzees perceive and process open and closed eyes diVerently.
Keywords
Chimpanzees, Configural processing, Eye, Inversion effect,
Introduction
Eyes are important to humans in understanding the visual perception, emotion, and communicative intention of other individuals (Emery 2000; Senju and Csibra 2008). Sensitivity to the eye area can be observed in infancy; neonates only a few days old looked at faces with open eyes longer than at faces with closed eyes when pictures of these two types of faces were presented simultaneously (Batki et al. 2000), and 4-month-old infants demonstrate specific brain activities that reflect the perception of eye contact (Farroni et al. 2002). Eyes are also considered to function as important stimuli for other animals, including serving as clues for predator detection, and experimental studies in birds have suggested that birds have specific sensitivity to eye-like stimuli (Gallup et al. 1971; Hampton 1994; Jones 1980; Scaife 1976a, b). Several studies of rhesus monkeys have directly measured their eye movements while viewing facial photographs (Dahl et al. 2007, 2009; Gothard et al. 2009; Guo et al. 2003; Keating and Keating 1982; Nahm et al. 1997). The results of these studies indicated that rhesus monkeys looked at eye areas more frequently than at any other facial parts depicted in the pictures.
Investigations of whether and how chimpanzees, the closest relatives of humans, look at the eyes of other individuals have produced mixed results. Naturalistic observations of chimpanzees have indicated that eye contact is within the repertoire of their friendly communicative behavior (de Waal 1982, 1989; Goodall 1986) and that mother–infant pairs engage in mutual eye contact (Bard et al. 2005). The results of an experimental investigation of infant chimpanzees have shown preferences for pictures of human faces with open eyes over those of human faces with closed eyes (Myowa-Yamakoshi et al. 2003). On the other hand, chimpanzees who succeeded in solving experimental cooperative tasks did not engage in eye contact with their partners, regardless of whether the partners were human or conspecific, during the process of cooperation (Hirata and Fuwa 2007; Hirata et al. in press). In other studies, chimpanzees showed bodily gestures to a human with closed eyes, suggesting that they did not understand that eyes function as the organ of vision (Kaminski et al. 2004; Povinelli et al. 2000), even though different behavioral experiments have demonstrated that chimpanzees were capable of understanding what others can and cannot see based at least on body and facial orientation (Hare et al. 2000; Tomasello et al. 2003). Researchers have argued that chimpanzees and other apes might not be predisposed to focus on eyes because their eyes are less visible than are human eyes, which are rendered highly visible by white sclera (Kaminski et al. 2004; Kobayashi and Kohshima 1997). No study, however, has directly investigated whether and to what extent chimpanzees attend to the open and closed eyes of other individuals.
Eyes are also important for the configural processing of facial stimuli (Diamond and Carey 1986). The definition of configural processing may vary somewhat among researchers, but Maurer et al. (2002) described it as the process involving sensitivity to the first-order relations (i.e., a nose above a mouth and two eyes above a nose) that specify the stimulus as a face, sensitivity to the second-order relations (i.e., spatial distance among features such as eyes, nose, and mouth) that specify differences among individuals in the spacing of features, and holistic processing that integrates facial features into a gestalt. A number of studies on humans have indicated that configural processing was disrupted when an upside-down face was viewed (e.g., Valentine 1988; Yin 1969), known as the face-inversion effect. Investigations of the face-inversion effect in non-human primates have produced mixed results. Some studies have indicated that face inversion did not influence recognition performance (Bruce 1982; Dittrich 1990; Gothard et al. 2004; Martin-Malivel and Fagot 2001; Weiss et al. 2001). However, other studies have shown superior perception of upright over inverted faces (Adachi et al. 2009; Parr et al. 1999; Tomonaga 1994).
Cognitive tasks with chimpanzees have suggested that they also show a face-inversion effect (Parr et al. 1998; Tomonaga 2007). Chimpanzees performed better in a matching-to-sample task involving upright compared to inverted presentations of chimpanzee and human faces (Parr et al. 1998). A chimpanzee performed better in searching for a target face that differed in orientation from distracters when the target had an upright orientation than when the target had an inverted orientation; this is referred to as the upright superiority effect (Tomonaga 2007). When only the eyes, nose, or mouth of a picture of a face was presented or when two of these were presented in combination in a subset of Tomonaga’s (2007) experiments, the upright superiority effect was observed only when pictures included the eye area, suggesting that the eye had an important role in the configural processing of faces.
Dahl et al. (2007, 2009) suggested the link between configural processing and attention to eyes. In their study, monkeys and humans looked at the eyes in upright faces more frequently than they looked at those in inverted faces (Dahl et al. 2009). Thus, the configural processing of faces appears to attract a high degree of attention to the eye region (Dahl et al. 2007, 2009). However, other studies have presented contradictory evidence (e.g., Gothard et al. 2009). Gothard et al. (2009) conducted experiments with monkeys that involved inverting and removing low spatial frequencies from facial images; it has been suggested that these manipulations also impair configural processing. The results showed that greater eye scanning by the monkeys occurred in response to both of these manipulations as well as in response to the original facial images. The cause of the inconsistency is not clear, and further comparative data are necessary.
We used an eye-tracking technique to assess how chimpanzees looked at eyes in a face to examine the role of the eye area in the perception of photographed faces. The first study of chimpanzee’s gazing patterns using an eye-tracking technique (Kano and Tomonaga 2009) showed that chimpanzees looked at faces more frequently than at other body parts, and a follow-up study (Kano and Tomonaga in press) indicated similarities and differences in the face-scanning patterns of chimpanzees and humans, including chimpanzees’ shorter fixation on and quicker scanning of the eyes and mouth. However, the effects of the state of the eyes (open or closed) and the orientation of the faces (upright and inverted) on chimpanzees’ scanning patterns are yet to be investigated. In the present study, chimpanzees were shown photographs of upright or inverted faces of conspecifics with open or closed eyes. We analyzed the data with regard to sensitivity to the eye, nose, and mouth areas and compared the time devoted to viewing the facial features of upright and inverted faces to explore whether chimpanzees processed open and closed eyes differentially.
Methods
Participants
Six chimpanzees (Pan troglodytes) participated in this study. These individuals were Loi (male, 13 years old), Zamba (male, 13 years old), Tsubaki (female, 13 years old), Mizuki (female, 12 years old), Misaki (female, 9 years old), and Natsuki (female, 3 years old). The participants lived in a group at the Great Ape Research Institute, Hayashibara Biomedical Laboratories, Inc.
Stimuli
Pictures of the faces of two infant chimpanzees, who were members of the same group as the six participants, were used as stimuli; the pictures depicted Hatsuka (female, 10 months old) and Iroha (female, 8 months old). Two types of facial pictures were prepared for each individual: one depicted a face with open eyes and the other depicted a face with closed eyes. The full faces of the models were recorded using a video camera, and a scene in which the individuals blinked was captured and used to depict a face with closed eyes. A scene directly before the chimpanzees started blinking was used to depict a face with open eyes. The facial expressions and the luminescence of the faces with open and closed eyes were identical, with the exception of the area surrounding the eyes. Faces were excised from their original background, converted to 500 pixels in height (430 pixels in width for one of the picture models and 490 pixels in width for the other), and placed in a gray frame (1024 × 768 pixels). The images of upright or inverted faces were placed in the upper or lower position in a frame. The absolute position of the eye area of the image of the upright face located at the lower position in the frame was identical to that of the image of the inverted face located at the upper position in the frame. A total of 16 pictures [2 picture models × 2 eye conditions (open and closed) × 2 facial orientations (upright and inverted) × 2 positions (upper and lower)] were prepared.
Apparatus
The experiment was conducted in an indoor room 2 m wide, 3 m long, and 2.5 m high. Human experimenters who were familiar to the chimpanzees remained in the room during testing. A Tobii (Stockholm, Sweden) T60 Eye Tracker was used to record the chimpanzees’ eye movements. The eye tracker was integrated with a 17-inch LCD display (1024 × 768 pixels), on which pictures were presented with Tobii Studio software. While each participant chimpanzee sat in front of the monitor on which the eye tracker was mounted, an experimenter stood beside the chimpanzee and positioned the participant’s face for measurement (Fig. 1). Another experimenter operated a PC (Dell M4400) that was connected to the eye tracker and controlled the calibration and presentation of the stimulus pictures. The experiment relied on voluntary participation by the chimpanzees, and they showed no negative emotional expressions, such as screams or grimacing, during testing.

Fig. 1 A chimpanzee viewing a monitor on which the eye tracker was mounted. Chimpanzees sat in front of a monitor at a distance of approximately 60 cm. A human experimenter held and supported the faces of the chimpanzees in a position appropriate for measurement
Procedure
1
Calibration. Calibration for each chimpanzee was achieved at the beginning of the session by showing a small video clip (160 × 120 pixels) at two calibration points. To verify calibration, two pictures consisting of a small circle, 30 pixels in diameter (1° visual angle at a 60-cm viewing distance) against a white background, were inserted during the test. The small circular picture appeared in a random position on a white background. Several pre-test sessions were also conducted to verify calibration. The chimpanzees’ fixation spots tended to concentrate around the small circular picture (Fig. 2). A measured fixation spot located closest to the center of the small circle was considered to represent the intention of chimpanzees to look at the small circle. An error of calibration was calculated as the distance between this measured fixation spot and the actual position of the small circular picture in the frame. The average error across participants was 11.7 pixels (SD = 11.0 pixels), equivalent to 0.40° (SD = 0.38°) of the visual angle of participants. The average errors for each chimpanzee were as follows: Loi, 16.6 pixels (SD = 10.8); Zamba, 8.8 pixels (SD = 10.3); Tsubaki, 4.4 pixels (SD = 7.1); Mizuki, 9.1 pixels (SD = 6.3); Misaki, 17.7 pixels (SD = 14.1); and Natsuki, 8.8 pixels (SD = 10.3). These errors were equivalent to visual angles of 0.15° ~ 0.66° at their viewing distance. The average errors on the basis of the two verification pictures presented during the test session (i.e., excluding pre-test sessions) were Loi, 8.2 pixels; Zamba, 4.9 pixels; Tsubaki, 5.2 pixels; Mizuki, 12.1 pixels; Misaki, 28.2 pixels; and Natsuki, 21.6 pixels.
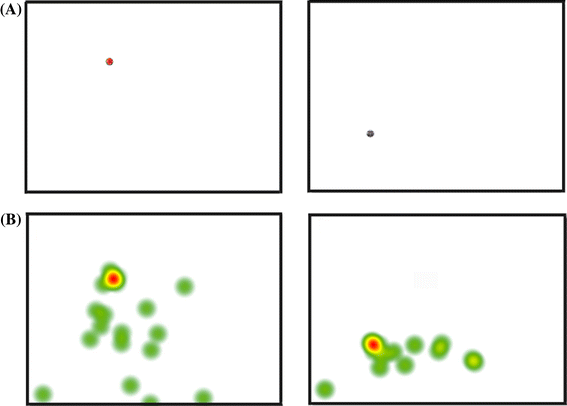
Fig. 2 Examples of pictures used to verify calibration and visualize fixation spots. a Examples of pictures used for the verification of calibration. A small circular picture was presented on a white background. b Illustration of fixation spots by six chimpanzees. Fixation densities while the six chimpanzees looked at Fig. 2a are superimposed on each picture to illustrate the fixation spots
2
Test. In addition to the 16 test pictures, 16 non-test pictures depicting landscapes or human activities familiar to the participants were inserted between test pictures to avoid the loss of interest associated with continuous presentation of test pictures. A total of 34 (16 test, 16 non-test, and 2 verification pictures) were presented in pseudorandom order in a session; the number of pictures in each of the four categories consisting of 2 eye conditions × 2 face orientations was identical in the first and second halves of each session. Presentations lasted 2.5 s for test pictures, 1 s for non-test pictures, and 1.5 s for pictures used to verify calibration. Data collection continued until each participant had viewed each of the 16 test pictures once. Five chimpanzees viewed all 16 test pictures in the first session. The remaining chimpanzee did not look at four of the 16 pictures in the first session, and a second session was conducted to collect data for those four pictures. Additional sessions using the same test pictures were actually conducted during subsequent days, but we encountered increasing amounts of missing data in these later sessions due to loss of interest in viewing the pictures. Therefore, we limited our analysis to the data set in which the participant first viewed the 16 test pictures.
Analysis
A fixation was scored using a Tobii fixation filter with a threshold radius of 35 pixels. Areas of interest (AOI) were determined for the entire face and for the eyes, nose, and mouth of each picture. The AOI for open eyes was slightly larger (20 pixels at the edge) than the border between the eyelid and eyeball. The same AOI was applied to closed eyes, so that the AOI for closed eyes covered exactly the same region in relation to the face as did the AOI for open eyes. The AOI for the nose was 20 pixels larger than the border connecting the top of the ridge of the nose where the protrusion of the chimpanzee nose/mouth area starts, the edge of the outer wall of the nostril, and the bottom of the nasal septum. The AOI for the mouth was 20 pixels larger than the border of the lips.
Several corrections were necessary to compare the fixation data for each part of the face because the AOIs for the eyes, nose, and mouth differed, and the viewing times for different pictures also differed. We used the method employed by Dahl et al. (2009) to obtain the scores for the viewing times for the eyes, nose, mouth, and entire face, although differences in the details of the methods might not allow for strict comparisons between the viewing times observed in our study and those observed by Dahl et al. (2009). The viewing-time scores were obtained by calculating the proportion of time spent viewing each part in relation to time spent viewing the entire image and then subtracting the proportion of the total image occupied by each part. For example, the viewing-time score of eyes was calculated as follows. First, the AOI of the eyes was divided by the total frame area to calculate the proportion of the area of the eyes accounted for by the AOI. Second, this proportion was subtracted from that of the fixation duration for the eyes, calculated by dividing the total time of gaze fixation within the AOI of the eyes for a certain picture by the total time of gaze fixation on that picture. The viewing-time scores for the nose, mouth, and face were calculated in the same way; we subtracted the proportion of the total frame area occupied by the target AOI from the proportion of the total fixation duration attributable to fixation on the target AOI for each picture.
Results
The chimpanzees looked at the face region, which covered approximately 20% of the area of the monitor, an average of 79.3% of the time (SD = 27.0). A repeated-measures ANOVA revealed no significant differences among the 16 test pictures in the proportion of time spent looking at the face region (F 15, 75 = 0.984, P = 0.48).
Participants paid particular attention to the eye, nose, and mouth regions (Fig. 3). The proportion of time devoted to looking at the eye area over the total time devoted to looking at the entire facial area was 3.92 times greater than chance (i.e., proportion of eye area over total face area) when participants looked at the face with open eyes (t-test, t 45 = 5.255, P < 0.001) and 2.19 times greater than chance when participants looked at the face with closed eyes (t-test, t 47 = 2.540, P = 0.014). The proportion of time devoted to looking at the nose area over the total time devoted to looking at the entire facial area was 4.03 times greater than chance (i.e., the proportion of the nose area over the total face area) when data for the face with open eyes and those for the face with closed eyes were pooled because the nose area was identical under these two conditions (t-test, t 92 = 4.529, P < 0.001). Similarly, the proportion of time devoted to looking at the mouth area over the total time devoted to looking at the entire facial area was 1.88 times greater than chance (i.e., the proportion of mouth area over the total face area) when data for the face with open eyes and those for the face with closed eyes were pooled (t-test, t 92 = 2.952, P = 0.004).
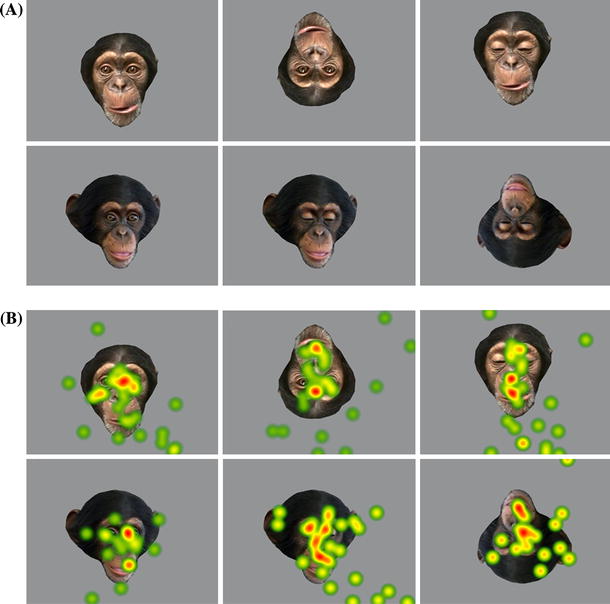
Fig. 3 Examples of the pictures presented and illustration of fixation spots. a Examples of face images. Pictures of chimpanzee faces with open and closed eyes were prepared and presented in an upright or inverted configuration at an upper or lower frame position. b Illustration of fixation spots by six chimpanzees. Fixation densities while six chimpanzees looked at each of the pictures in Fig. 3a are superimposed on each picture to illustrate the fixation spots. Colors from green to yellow to red represent lower to higher fixation densities; this is a rough illustration for each picture, and accurate comparisons between pictures must use raw fixation data
Figure 4 shows the viewing-time scores for the eyes, nose, and mouth for four categories of pictures. Average viewing-time scores for the eyes, nose, and mouth were as follows: 0.41 (SD = 0.31), 0.17 (SD = 0.31), and 0.07 (SD = 0.16), respectively, when participants looked at upright faces with open eyes; 0.08 (SD = 0.16), 0.16 (SD = 0.34), and 0.15 (SD = 0.21), respectively, when they looked at upright faces with closed eyes; 0.16 (SD = 0.23), 0.09 (SD = 0.22), and 0.06 (SD = 0.12), respectively, when they looked at inverted faces with open eyes; and 0.22 (SD = 0.31), 0.08 (SD = 0.14), and 0.13 (SD = 0.21), respectively, when they looked at inverted faces with closed eyes.
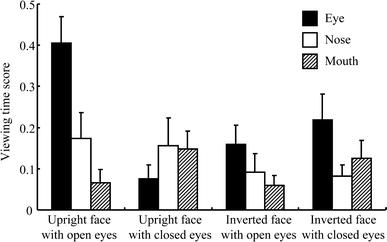
Fig. 4 Viewing-time scores for the eye, nose, and mouth areas of each category of pictures. The error bar indicates standard error. A difference greater than 0 in viewing times indicates that the area was looked at more than would be predicted by a uniform viewing strategy
The viewing-time score for eyes were higher than were those for the nose and mouth when participants looked at upright faces with open eyes (F 2,10 = 4.086, P = 0.050). The differences in the viewing-time scores for the eyes, nose, and mouth were not significant when participants looked at other types of faces (upright faces with closed eyes, F 2,10 = 0.393, P = 0.685; inverted faces with open eyes, F 2,10 = 0.855, P = 0.454; inverted faces with closed eyes, F 2,10 = 1.576, P = 0.254).
We found similar distributions of the average fixation durations for each part in each category of pictures during the 2.5-s picture presentations. The average durations for looking at eyes, nose, and mouth were as follows: 0.99 s (SD = 0.77), 0.51 s (SD = 0.72), and 0.16 s (SD = 0.27), respectively, when participants looked at upright faces with open eyes; 0.22 s (SD = 0.40), 0.28 s (SD = 0.51), and 0.32 s (SD = 0.48), respectively, when they looked at upright faces with closed eyes; 0.41 s (SD = 0.56), 0.23 s (SD = 0.51), and 0.13 s (SD = 0.23), respectively, when they looked at inverted faces with open eyes; and 0.43 s (SD = 0.64), 0.16 s (SD = 0.22), and 0.27 s (SD = 0.46), respectively, when they looked at inverted faces with closed eyes.
Regarding the viewing-time score for eyes, repeated-measures analysis of variance (ANOVA) with the main factors of eye condition (open and closed), orientation of face (upright and inverted), position of face in a frame (upper and lower), and picture model revealed no significant effect for any of the main factors (eye condition, F 1,5 = 4.221, P = 0.095; orientation of face, F 1,5 = 1.701, P = 0.249; position, F 1,5 = 2.377, P = 0.184; picture model, F 1,5 = 0.003, P = 0.96) or for any type of interaction, with the exception of one significant interaction between eye condition and orientation (F 1,5 = 27.696, P = 0.003; F 1,5 < 1.800, P > 0.237 for all other types of interactions). Subsequent analyses to examine simple main factors showed that chimpanzees looked at the open eyes of upright faces significantly longer than they looked at the open eyes of inverted faces (F 1,5 = 8.817, P = 0.031), but we found no significant difference in time spent looking at the eye areas of the upright versus the inverted faces with closed eyes (F 1,5 = 2.942, P = 0.147). Similarly, the chimpanzees looked at the open eyes of upright faces significantly longer than they looked at the closed eyes of upright faces (F 1,5 = 15.704, P = 0.011), but the difference between open and closed eyes was not significant for inverted faces (F 1,5 = 0.521, P = 0.503).
Repeated-measures ANOVAs revealed no significant effect for any of the main factors (F 1,5 < 1.661, P > 0.254) and no interactions of factors (F 1,5 < 2.870, P > 0.151) with regard to the viewing-time scores for the nose. Repeated-measures ANOVAs revealed no significant effect for any of the main factors (F 1,5 < 1.662, P > 0.254) and no interactions of factors (F 1,5 < 4.376, P > 0.091) regarding the viewing-time scores for the mouth. Repeated-measures ANOVAs revealed no significant effect for any of the main factors (F 1,5 < 3.235, P > 0.132) and no interactions of factors (F 1,5 < 2.997, P > 0.144) regarding the viewing-time scores for the entire face.
The difference in the time devoted to looking at open eyes in upright and inverted faces was not due to the influence of the absolute positions of the eyes within the frame. First, no main effect for the position of the images or for any type of interaction regarding the position was detected, as described earlier. Thus, the time that chimpanzees spent looking at the eye areas was not significantly influenced by the absolute positions of the images within the frame. Second, viewing times were significantly different even when the absolute positions of the eye areas of the upright and inverted face images were identical. That is, the absolute position of the eye area in the image of the upright face located at the lower position in the frame was identical to that in the image of the inverted face located at the upper position in the frame, but we found a significant difference in the amounts of time spent looking at the eye areas of these images (paired t-test, t 11 = 2.412, P = 0.035).
The difference in the scores for viewing times was not caused by the order in which pictures were presented. When the presentation of pictures was organized according to whether the eyes were open or closed and whether the face was upright or inverted, the number of pictures in each of the four categories (upright face with open eyes, inverted face with open eyes, upright face with closed eyes, and inverted face with closed eyes) was the same in the first and second halves of one session for all subjects. In addition, whether the pictures in each of these categories were presented in the first or second half of a session did not have a significant effect on the scores for the time spent viewing the eye areas (F 1,5 = 0.002, P = 0.963).
Despite some individual variations in looking patterns, three of the six participants (Loi, Tsubaki, and Natsuki) spent the longest amount of time viewing the open eyes in upright faces compared to the time they spent viewing the other configurations (3 features: eyes, nose, and mouth × 4 categories of pictures). The fourth individual (Misaki) spent the most time viewing the nose in upright faces with closed eyes and the second longest time viewing the open eyes in upright faces. The fifth individual (Mizuki) spent the longest time viewing the nose in upright faces with open eyes and the third longest time viewing the open eyes in upright faces. The last individual (Zamba) spent the most time viewing the mouth in upright faces and the fourth longest time viewing the open eyes in upright faces. The age of one of the six participants (Natsuki, 3 years old) differed significantly from that of the other individuals (9–13 years old), but data obtained from this participant were entirely within the range of the data obtained from the other individuals, and t-tests revealed no significant differences between the data for Natsuki and those for the other individuals when viewing-time scores were compared by feature for each of the four categories of pictures (0 < t 22 < 1.113, P > 0.278, or −1.488 < t 22 < 0, P > 0.151).
The analysis of the number of bouts fixating on the eye, nose, and mouth areas demonstrated a tendency similar to that found with respect to fixation duration. Figure 5 shows the average number of fixation bouts for each feature in each category of pictures. The number of fixation bouts for the eyes was larger than that for the nose and mouth when participants looked at upright faces with open eyes (F 2,10 = 7.327, P = 0.011). The differences in the number of fixation bouts for the eyes, nose, and mouth were not significant when participants viewed other types of faces (upright faces with closed eyes, F 2,10 = 0.154, P = 0.859; inverted faces with open eyes, F 2,10 = 1.118, P = 0.365; inverted faces with closed eyes, F 2,10 = 0.913, P = 0.432).
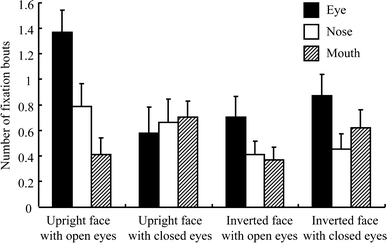
Fig. 5 Average number of fixation bouts for the eye, nose, and mouth areas of each category of pictures. The error bar indicates standard error
The following were the average bout lengths for the eyes, nose, and mouth, respectively, in each category of pictures: 740, 642, and 393 ms for upright faces with open eyes; 369, 425, and 451 ms for upright faces with closed eyes; 573, 561, and 351 ms for inverted faces with open eyes; and 493, 353, and 439 ms for inverted faces with closed eyes. Overall, the length of a fixation bout for the eyes, nose, and mouth did not differ significantly (F 2,188 = 1.493, P = 0.227). The bout length for the eyes was longer when participants viewed open eyes than when they viewed closed eyes even though the effect of eye condition (open or closed) was not statistically significant (F 1,80 = 2.973, P = 0.089). In addition, the effect of orientation was also not significant (F 1,80 = 0.027, P = 0.869). The effects of eye condition and orientation were not significant with respect to bout length for the nose and mouth (F 1,80 < 1.701, P > 0.198). Thus, the difference in total duration shown in Fig. 4 was caused primarily by the difference in the number of fixation bouts, shown in Fig. 5.
Discussion
Our findings showed that chimpanzees paid great attention to characteristic parts within faces such as the eye, nose, and mouth areas when viewing photographs of conspecific faces. More specifically, they looked at the eyes longer than at other parts of the faces when photographs of upright faces with open eyes were presented. We employed eye-tracking techniques that allowed us to examine participants’ natural gazing patterns. The results obtained thus reflected the chimpanzees’ spontaneous strategies for looking at photographs of faces in the absence of any artifacts caused by human training and reinforcement for responding differentially to the visual stimuli. Previous studies have indicated that both macaques and humans looked at eye areas more frequently than at any other parts of photographs of conspecific faces (e.g., Hainline 1978; Keating and Keating 1982). The present results suggest that particular attention to the eyes within a face in an upright orientation represents a spontaneous face-scanning strategy shared among monkeys, apes, and humans.
Differences in the patterns of looking at eyes became clearer when data for upright and inverted faces were compared. We manipulated the factor of face orientation (upright/inverted) following reports by a number of studies on the face-inversion effect (e.g., Maurer et al. 2002). Dahl et al. (2009) reported that in humans and macaques, the frequency with which the eye areas of conspecific individuals were looked at was higher when participants viewed upright faces with open eyes than when they viewed inverted faces with open eyes. Those authors concluded that these results constituted evidence in support of the configural processing of upright faces. The results of the open-eye condition of the present study are similar to those obtained by Dahl et al. (2009). Inverting a face, the diagnostic marker of holistic processing, affected the scanning of facial features in our study with chimpanzees as well.
We also manipulated the factor of eye condition (open/closed) following speculation that chimpanzees are not predisposed to focus on eyes that emerged after a previous study reported that chimpanzees did not discriminate between humans with open and closed eyes in behavioral tasks (Kaminski et al. 2004). Our results showed a significant interaction of eye condition and orientation. It is likely that configural processing and the different saliency of open and closed eyes contributed to the interaction of these factors. Open and closed eyes play a role in the ability to extract holistic information from the face, in that faces with closed eyes do not appear to be processed holistically. However, no data on humans obtained under the same conditions as those used in this study have been presented; thus, further comparative research is needed to specify the processes affecting the interaction of the two factors.
Our data are at least suggestive of the possibility that the chimpanzees perceived and processed open and closed eyes differently. This finding is consistent with that of a previous study performed by Myowa-Yamakoshi et al. (2003), which illustrated that chimpanzee infants discriminated between pictures of human faces with open and closed eyes. The exact location of the gazes of the chimpanzees was not assessed in the previous study, but the present study confirmed that chimpanzees viewed the open and closed eyes of pictures of faces and that they looked at open eyes more frequently than they looked at closed eyes when pictures were presented in an upright orientation.
These results provide evidence contradicting prior speculation that chimpanzees are not predisposed to focus on eyes. Despite their predisposition to look at eyes, chimpanzees might lack an accurate understanding that others cannot see with closed eyes. Eye-tracking studies with macaque monkeys have shown that participants looked at the eye areas of facial pictures of conspecifics more frequently (e.g., Dahl et al. 2009). However, monkeys tend to avoid directly gazing at the eyes of other individuals in actual interactions because staring at eyes functions as a threat (e.g., de Waal 1989; Gomez 1996; Redican 1975). Thus, the attention paid by monkeys to the eye areas in photographs is considered to be inhibited in encounters with real individuals. Staring at eyes does not function as an apparent threat in chimpanzees, but some avoidance mechanisms might operate in interactions with real individuals. Chimpanzees did not employ eye contact when they engaged in coordinated actions with cooperative partners in experimental cooperative tasks (Hirata and Fuwa 2007; Hirata et al. in press). Taken together, these studies suggest that eyes might serve different roles for monkeys, chimpanzees, and humans in natural social interactions, despite their shared sensitivity to eye areas, as measured by reactions to picture presentations. Future research will need to address these discrepancies by testing the difference between fixations on still image and those on real individuals. Another consideration concerns the possible absence of an adequate experimental procedure for probing chimpanzees’ understanding of eyes. Indeed, a few apes have demonstrated some sensitivity to the state of the eyes (i.e., open or closed) in situations involving requests of humans, despite the total samples providing evidence to the contrary (Gomez1996; Kaminski et al. 2004).
Our study has opened a new window to investigating how chimpanzees see the world by introducing eye-tracking techniques. Future directions include investigating developmental changes in face-viewing patterns; the effects of the age, sex, and familiarity of the individual depicted in the photograph; and the effects of more explicit manipulations of photographs that involve disturbances to configural processing. Studies with human subjects have shown that configural processing develops slowly (Mondloch et al. 2002), that the age of and familiarity with the models affect recognition performance (Anastasi and Rhodes 2005; Collishaw and Hole 2000), and that certain types of spatial filtering of the photographs affect configural and featural processing (Goffaux et al. 2005). The limited number of individuals housed at the institute where the present study was conducted did not allow further considerations of such issues as developmental changes or the effect of models with different characteristics. One of the participants in the present study was much younger (3 years) than the others (9–13 years), but the data obtained from the 3-year-old individual were within the range of those obtained from other individuals, at least in the current investigation. Data from a greater number of participants and from younger individuals may reveal finer developmental distinctions. We used photographs of individuals who were familiar to the participants rather than of the participants themselves to avoid factors related to familiarity and self/other considerations. This resulted in using two infants as photograph models. In other words, future research can intentionally manipulate these aspects. Further studies using this paradigm might help in obtaining evidence pertinent to chimpanzees’ understanding of eyes, the internal states of others, and other domains of social cognition.
In conclusion, the present study evaluated chimpanzees’ attention to the eyes, nose, and mouth in facial photographs and indicated their particular sensitivity to the areas around the open eyes of upright faces. These results suggest their recognition of the spatial configuration of the face and their differential perceptual processing of open and closed eyes; these processes constitute a basic step toward higher-level social cognition.
Acknowledgments
This work was financially supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#20220004 and #20680015). We thank Kazuo Fujita, Shoji Itakura, and Masako Myowa-Yamakoshi for their support in conducting the experiment, Robert W. Shumaker for helpful comments on the manuscript, and the staff of the Great Ape Research Institute, Hayashibara Biochemical Laboratories, Inc. for support in conducting the experiment and for caring for the chimpanzees. All chimpanzees were cared for according to the Guide for the Care and Use of Laboratory Animals of Hayashibara Biochemical Laboratories, Inc. and the guidelines established by the Primate Society of Japan. The authors declare that they have no conflict of interest.
References
- Adachi I, Chou DP, Hampton RR (2009) Thatcher effect in monkeys demonstrates conservation of face
perception across primates.
Curr Biol 19:1270–1273
- Anastasi J, Rhodes MG (2005) An own-age bias in face recognition for children and older adults.
Psychon Bull Rev 12:1043–1047
- Bard K, Myowa-Yamokoshi M, Tomonaga M, Tanaka M, Costal A, Matsuzawa T (2005) Group differences in
the mutual gaze of chimpanzees
(Pan troglodytes). Dev Psychol 41:616–624
- Batki A, Baron-Cohen S, Wheelwright S, Connellan J, Ahluwalia J (2000) Is there an innate gaze
module? Evidence from human
neonates. Inf Behav Dev 23:223–229
- Bruce C (1982) Face recognition by monkeys: absence of an inversion effect. Neuropsychologia 20:515–521
- Collishaw SM, Hole GJ (2000) Featural and configurational processes in the recognition of faces of
different familiarity.
Perception 29:893–909
- Dahl CD, Logothetis NK, Hoffman KL (2007) Individuation and holistic processing of faces in rhesus
monkeys. Proc R Soc B 274:2069–2076
- Dahl CD, Wallraven C, Bulthoff HH, Logothetis NK (2009) Humans and macaques employ similar
face-processing strategies. Curr
Biol 19:509–513
- De Waal FBM (1982) Chimpanzee politics: power and sex among apes. Jonathan Cape, London
- De Waal FBM (1989) Peacemaking among primates. Harvard University Press, Cambridge
- Diamond R, Carey S (1986) Why faces are and are not special: an effect of expertise. J Exp Psychol
115:107–117
- Dittrich W (1990) Representation of faces in longtailed macaques (Macaca fascicularis).
Ethology 85:265–278
- Emery NJ (2000) The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci
Biobehav Rev 24:581–604
- Farroni T, Csibra G, Simion F, Johnson MH (2002) Eye contact detection in humans from birth. Proc
Natl Acad Sci USA 99:9602–9605
- Gallup GG, Nash RF, Ellison AL (1971) Tonic immobility as a reaction to predation: artificial eyes as a fear stimulus for chickens. Psychon Sci 23:79–80
- Goffaux V, Hault B, Michel C, Vuong QC, Rossion B (2005) The respective role of low and high spatial
frequencies in supporting
configural and featural processing of faces. Perception 34:77–86
- Gomez JC (1996) Non-human primate theories of (non-human primate) minds: some issues concerning the origins of mind-reading. In: Carruthers P, Smith PK (eds) Theories of theories of mind. Cambridge University Press, Cambridge, pp 330–343
- Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Harvard University Press, Cambridge
- Gothard KM, Erickson CA, Amaral DG (2004) How do rhesus monkeys (Macaca mulatta) scan faces
in a visual paired comparison task? Anim Cogn 7(1):25–36
- Gothard KM, Brooks KN, Peterson MA (2009) Multiple perceptual strategies used by macaque monkeys for
face recognition. Anim
Cogn 12:155–167
- Guo K, Robertson RG, Mahmoodi S, Tadmor Y, Young MP (2003) How do monkeys view faces? A study of eye
movements. Exp Brain
Res 150:363–374
- Hainline L (1978) Developmental changes in visual scanning of face and nonface patterns by infants.
J Exp Child Psychol 25:90–115
- Hampton RR (1994) Sensitivity to information specifying the line of gaze of humans in sparrows (Passer
domesticus). Behaviour 130:41–51
- Hare B, Call J, Agnetta B, Tomasello M (2000) Chimpanzees know what conspecifics do and do not see.
Anim Behav 59:771–785
- Hirata S, Fuwa K (2007) Chimpanzees (Pan troglodytes) learn to act with other individuals in
a cooperative task. Primates 48:13–21
- Hirata S, Morimura N, Fuwa K (in press) Intentional communication and comprehension of the partner’s role in experimental cooperative tasks. In: Lonsdorf EV, Ross SR, Matsuzawa T (eds) The mind of the chimpanzees: ecological and experimental perspectives. The University of Chicago Press, Chicago
- Jones RB (1980) Reactions of male domestic chickens to two-dimensional eye-like shapes. Anim Behav
28:212–218
- Kaminski J, Call J, Tomasello M (2004) Body orientation and face orientation: two factors
controlling apes’ begging behavior
from humans. Anim Cogn 7:216–223
- Kano F, Tomonaga M (2009) How chimpanzees look at pictures: a comparative eye-tracking study. Proc R
Soc B 276:1949–1955
- Kano F, Tomonaga M (in press) Face scanning in chimpanzees and humans: continuity and discontinuity. Anim Behav in press
- Keating CF, Keating EG (1982) Visual scan patterns of rhesus monkeys viewing faces. Perception
11:211–219
- Kobayashi H, Kohshima S (1997) Unique morphology of the human eye. Nature 6635:767–768
- Martin-Malivel J, Fagot J (2001) Perception of pictorial human faces by baboons: effects of stimulus orientation on discrimination performance. Anim Learn Behav 29:10–20
- Maurer D, Le Grand R, Mondloch CJ (2002) The many faces of configural processing. Trends Cogn Sci
6:255–260
- Mondloch CJ, Le Grand R, Maurer D (2002) Configural face processing develops more slowly than
featural processing. Perception
31:553–566
- Myowa-Yamakoshi M, Tomonaga M, Tanaka M, Matsuzawa T (2003) Preference for human direct gaze in infant chimpanzees (Pan troglodytes). Cognition 89:B53–B64
- Nahm FKD, Perret A, Amaral DG, Albright TD (1997) How do monkeys look at faces? J Cogn Neurosci
9:611–623
- Parr LA, Dove T, Hopkins WD (1998) Why faces may be special: evidence of the inversion effect in
chimpanzees. J Cogn Neurosci
10:615–622
- Parr LA, Winslow JT, Hopkins WD (1999) Is the inversion effect in rhesus monkeys face-specific? Anim
Cogn 2:123–129
- Povinelli DJ, Bering JM, Giambrone S (2000) Toward a science of other minds: escaping the argument
by analogy. Cogn Sci 24:509–541
- Redican WK (1975) Facial expressions in nonhuman primates. In: Rosenblum LA (ed) Primate behavior, vol 4. Academic Press, New York, pp 103–194
- Scaife M (1976a) The response to eye-like shapes by birds I: The effect of context, a predator and a
strange bird. Anim Behav
24:195–199
- Scaife M (1976b) The response to eye-like shapes by birds II: the importance of staring, pairedness
and shape. Anim Behav
24:200–206
- Senju A, Csibra G (2008) Gaze following in human infants depends on communicative signals. Curr Biol
18:668–671
- Tomasello M, Call J, Hare B (2003) Chimpanzees understand psychological states—the question is
which ones and to what extent.
Trends Cogn Sci 7:153–156
- Tomonaga M (1994) How laboratory-raised Japanese monkeys (Macaca fuscata) perceive rotated
photographs of monkeys: evidence for an inversion effect in face perception. Primates 35:155–165
- Tomonaga M (2007) Visual search for orientation of faces by a chimpanzee (Pan troglodytes):
face-specific upright superiority and the role of facial configural properties. Primates 48:1–12
- Valentine T (1988) Upside-down faces: a review of the effects of inversion on face recognition. Br J
Psychol 79:471–491
- Weiss DJ, Kralik JD, Hauser MD (2001) Face processing in cotton-top tamarins (Saguinus oedipus).
Anim Cogn 4:191–205
- Yin RK (1969) Looking at upside down faces. J Exp Psychol 81:141–145