Measuring heart rate in captive chimpanzees without anesthesia
Satoshi Hirata, Etsuko Nogami, Toshifumi Udono
Abstract
Heart rate measurements can be useful for the monitoring of both physical and mental condition in humans and nonhuman animals. Yet, information about heart rates in chimpanzees, who are phylogenetically the closest species to humans, is scarce. Existing data on chimpanzee heart rates have mainly been collected from chimpanzees under anesthesia. To address this issue, we conducted electrocardiogram recordings in captive chimpanzees under normal conditions without anesthesia based on positive reinforcement training. We obtained a total of 771 recordings from 35 individuals (22 males and 13 females, 14–53 years old) with no cardiac problems. The females had a higher heart rate than the male chimpanzees, and heart rate decreased as a function of age. In addition, heart rate was lower in the morning and increased during the day. Overall, the mean heart rate of adult males was 86.5 beats/min, and that of female chimpanzees 106.4 beats/min. Our data could serve as a reference point for future research and health-based monitoring of chimpanzee heart rates.
Introduction
Heart rate is an important indicator of the physical and mental state of both humans and nonhuman animals. Although several studies have described the collection of heart rate data from chimpanzees (Pan troglodytes), the closest living relatives of humans, the majority of these data were collected from individuals who were under anesthesia, as a result of the challenges associated with obtaining the data from awake chimpanzee. Atencia et al. (2015) described the characteristics of electrocardiograms (ECGs) of 100 chimpanzees housed at an African sanctuary. The data were obtained during physical assessments made when the chimpanzees were under anesthesia. The mean heart rate of individuals under 10 years of age (n = 51) was 71 beats/min (bpm), and that of adult individuals (10 years of age or older, n = 49) was 63 bpm. Atencia et al. (2017) investigated the effects of different types of anesthesia on chimpanzee heart rate in a total of 176 individuals from three African sanctuaries. Drane et al. (2020) compared ECG characteristics and echocardiographic data in a total of 341 chimpanzees from three African sanctuaries and two zoos. Additional anecdotal findings and reports involved smaller sample sizes (Erickson and Olsen 1985; Lonsdorf et al. 2014; Drane et al. 2021). Taken together, the existing data indicate that the mean heart rate of chimpanzees under anesthesia is approximately 68–88 bpm, with a range from approximately 40 to 100 bpm. However, these data were obtained using various combinations of pharmaceutical anesthetic agents.
In the 1980s, Boysen and Bernston (1986; 1989) measured the heart rate of a young chimpanzee in a normal awake condition without anesthesia to investigate the effects of visual stimuli on heart rate. The study was conducted when the subject female chimpanzee was 3.5 years old and when she was 4.5 years old. The heart rate of this young chimpanzee was around 80–120 bpm. More recently, Serrano-Finetti et al. (2023) conducted a study on heart rate in four awake adult male chimpanzees without anesthesia. In their study, a caregiver wore a special glove with electrodes embedded into the fingertips. By touching a chimpanzee in a normal awake state, the glove allowed the caregiver to obtain ECG data. The mean reported heart rate was 65 bpm, but detailed information such as the range and SD were not reported.
Bonobos, along with chimpanzees, belong to the genus Pan. Two studies have measured heart rate in bonobos in normal awake conditions. Olds et al. (2023) used KardiaMobile sensors to obtain ECG data from six awake bonobos without anesthesia, and reported their heart rate to be 87 bpm (range = 60–118 bpm). Danforth et al. (2023) used the petMap device to measure blood pressure and heart rate in captive bonobos at eight North American zoos. A total of 2845 good-quality recordings were obtained from 36 individuals, and the mean heart rate was 110 bpm (range = 75–187 bpm).
Because of difficulties associated with the placement of recording probes, such as electrodes, onto the skin of awake chimpanzees, researchers have attempted to develop non-contact methods for measuring heart rate in this species. For example, Iwata et al. (2023) used a millimeter-wave radar device to measure heart rates in two captive chimpanzees, which were verified by simultaneously obtained contact ECG data. The millimeter-wave radar data were consistent with the ECG data. However, the chimpanzees were under anesthesia during data collection, and the result of the heart rate calculation was not reported.
Wang et al. (2023) obtained non-contact heart rate measurements via a new image-analysis technique in which fine-scale cardiopulmonary signals are extracted according to the cyclic motion of the subject’s body. They verified data collected from seven chimpanzees by simultaneously measuring heart rate using classic photoplethysmography (PPG) finger sensors. The authors concluded that the results of the two methods (image analyses and PPG) were in agreement. However, the range of the estimated heart rates was from 140 to 170 bpm, which is far higher than that measured for chimpanzees under or not under anesthesia. In our experience, PPG-based measurements of heart rate often yield unreliable data in chimpanzees because of their highly pigmented and thick skin.
The most reliable methods for monitoring heart rate are contact-based measurements such as ECG or echocardiography. In the development of new non-contact heart rate measurement techniques, accurate heart rate data collected in normal awake conditions without anesthesia are essential as reference points. Accordingly, we collected ECG data from a total of 35 captive chimpanzees in normal awake conditions following their husbandry training with positive reinforcement.
Methods
The study participants were chimpanzees at Kumamoto Sanctuary (Wildlife Research Center, Kyoto University). Care staff conducted husbandry training with the chimpanzees to encourage them to permit the placement of electrodes on the skin of their chest. A portable ECG device (HCG-901) with external electrodes (HCG-901-LEAD; OMRON) was used. We modified the tip of the electrodes for placement on a chimpanzee’s chest (see supplementary materials for details). We conducted the training during individual feeding periods, at which time each chimpanzee was temporarily kept isolated in an indoor or outdoor room. Each recording session was ideally 2 min long, although the participant chimpanzees could leave the site at any point. Small pieces of fruit were given to the participant chimpanzees as positive reinforcement for sitting still and permitting the recordings to be made (Fig. 1). In the present study, we analyzed data recorded from 16 November 2013 to 31 May 2023, during which time a total of 1909 recordings were obtained from 55 individual chimpanzees.
The heart rate calculations were conducted using HCG-SOFT-CL1 (OMRON) bundled software (Fig. 2). If the data were too noisy to calculate the heart rate by using the software, in cases where it was possible to count the R waves for more than 15 consecutive seconds (not including an extrasystole), we made the calculations manually.
As we sought to report the heart rate of visibly healthy chimpanzees in the present paper, we excluded chimpanzees who had been infected with hepatitis C during a previous biomedical experiment (Hirata et al. 2022) from the analysis. We also excluded individuals who had been diagnosed with cardiac problems during physical examinations. When two or more recordings were conducted in a day, the recording with the best quality was used for the analyses.
The final dataset was collected from 35 individuals, as follows: 562 recordings (5–46 recordings per individual) from 22 males, 14–53 years old (mean = 31.0, SD = 8.2); 209 recordings (2–40 recordings per individual) from 13 females, 5–47 years old (mean = 32.4, SD = 10.9).
We used a linear mixed model to assess the effects of sex, age, and time of day of the recording session. In this model, heart rate was the response variable and sex, age, and time of day were fixed effects. The identity of each individual was added as a random effect to control for repeated measures. We used the likelihood ratio test to examine the significance of the effects. We used the R package lmer4 version 4.1.2 for the statistical analyses.
Results and discussion
Figures 3 and 4 show scatter plots of heart rate as a function of age and the time of day, according to sex. The linear mixed model showed that the effects of sex, age, and the time of day were all significant (sex, χ2 = 19.28, p < 0.001; age, χ2 = 15.01, p < 0.001; time of a day, χ2 = 17.67, p < 0.001). Females had a higher heart rate than male chimpanzees, and heart rate decreased as a function of age. In addition, heart rate was lower in the morning and increased during the day.
Table 1 shows a summary of the data from the adult chimpanzees (excluding juveniles ≤ 10 years old). The data were from 22 males aged 14–53 years (562 recordings in total) and ten female chimpanzees aged 15–47 years (192 recordings in total). Because of the daily husbandry schedule, recordings from the males were mainly conducted in the morning and those from the female chimpanzees were mainly conducted in the afternoon. Thus, the observed difference in male and female heart rates was likely influenced by the time of day of measurement in these two groups.
Wang et al. (2023), using a new type of image analysis technology, estimated the heart rate of one juvenile (9 years old) and six adult (19–44 years old) captive chimpanzees, as ranging from 140 to 170 bpm, which is higher than the range observed in the present study. Given this difference, additional work is needed to verify the accuracy of the method used by Wang et al. (2023).
Our heart rate values were higher than those reported by Serrano-Finetti et al. (2023), who measured heart rate in four captive male chimpanzees without the use of anesthesia and reported a mean value of 65 bpm. However, this value (65 bpm) is within the 95% probability range (64–108 bpm) of our data from male chimpanzees. Serrano-Finetti et al. (2023) did not indicate the time of day at which the measurements were taken, which was an important variable in the present study. The two studies on heart rate measured in captive bonobos under normal conditions without anesthesia reported values that were similar to and within the range of our results (Olds et al. 2023; Danforth et al. 2023). Thus, heart rates of the two species of the genus Pan appear to be similar.
In humans, females have a higher heart rate than males, and heart rate decreases as a function of age (Bonnermeier et al. 2003; Sagie et al. 1992; Singh et al. 1999). In addition, heart rate is lower in the morning and increases during the day (Vandewalle et al. 2007). Our results from chimpanzees showed a comparable trend, suggesting that similar factors affect heart rate in both chimpanzees and humans. We did not collect ECG data from the chimpanzees in the evening or at night because these periods did not align with their routine daily care. In humans, heart rate starts to decrease at night in the awake state, and decreases to its lowest level during sleep (Vandewalle et al. 2007). Future research is needed to determine whether or not chimpanzees show a similar circadian trend in heart rate.
Our results could serve as a reference point for the future development of non-contact methods for measuring heart rate in chimpanzees. It should be noted, however, that the heart rates reported in the present study may differ from those in chimpanzees in a naturalistic, relaxed condition. The presence of the caregiver and the electrode placement process might have induced an increased arousal level in the chimpanzees, which could have increased their heart rate. However, because we repeated the measurement process in the same chimpanzees, they likely experienced some degree of habituation. Accordingly, some of the data presented in the scatter plots in Figs. 3 and 4 likely represent the heart rate in a normal relaxed condition.
Limitations of our method are the use of dry electrodes instead of pre-gelled electrodes, and modification in the placement of the ground electrode. These modifications are associated with drawbacks such as lowering the amplitude in the ECG and potentially introducing deviations in the general characteristics of the waveform. Therefore, the waveforms cannot be used to measure features other than the heart rate, such as amplitude of the QRS complex. However, our method does not impose a problem in the determination of heart rate because measurements of the R-R interval were reliable and sufficient for this purpose. Heart rate measurements could be used to compare healthy with sick individuals, for example to check for the occurrence of arrhythmia. The technique described here could also be used for heart rate monitoring in individuals undergoing treatment with beta blockers and other types of drugs.
Of note, as illustrated by the images shown in Fig. 1, is that application of the method described here was possible because of the relationships between the skilled staff and the target chimpanzees. Regulations by other facilities may prohibit this style of interaction between staff and chimpanzees for safety issues. Use of a mobile device such as KardiaMobile offers a safer training option (Cloutier Barbour et al. 2020). Validating various different methods and selecting a safer method for use will promote the health monitoring of great apes in captivity.
Acknowledgements
This study was supported financially by the Great Ape Information Network. We thank Sydney Koke from Edanz and Kristin Havercamp for editing a draft of this manuscript.
References
- Atencia R, Revuelta L, Somauroo JD, Shave RE (2015) Electrocardiogram reference intervals for clinically normal wild-born chimpanzees (Pan troglodytes). Am J Vet Res 76:688–693.
- Atencia R, Stöhr EJ, Drane AL, Stembridge M, Howatson G, Del Rio PRL, Feltrer Y, Tafon B, Redrobe S, Peck B, Eng J, Unwin S, Sanchez CR, Shave RE (2017) Heart rate and indirect blood pressureresponses to four different field anesthetic protocols in wildborn captive chimpanzees (Pan troglodytes). J Zoo Wildl Med 48:636–644.
- Bonnermeier H, Wiegand UKE, Brandes A, Kluge N, Katus HA, Richardt G, Potrantz J (2003) Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging gender on heart rate variability. J Cardiovasc Electrophysiol 14:791–799
- Boysen ST, Berntson GG (1986) Cardiac correlates of individual recognition in the chimpanzee (Pan troglodytes). J Comp Psychol 100:321–324
- Boysen ST, Berntson GG (1989) Conspecific recognition in the chimpanzee (Pan troglodytes): cardiac responses to significant others. J Comp Psychol 103:215–220
- Cloutier Barbour C, Danforth MD, Murphy H, Sleeper MM, Kutinsky I (2020) Monitoring great ape heart health through innovative electrocardiogram technology: training methodologies and welfare implications. Zoo Biol 39:443–447
- Danforth MD, Clyde VL, Jourdan B, Korman R, Beehler L, Wann S, Bapodra P, Murphy H, Gerlach T, Rich S, Thorgerson AM (2023) Blood pressure monitoring in zoologically managed bonobos (Pan paniscus). Am J Primatol.
- Drane AL, Atencia R, Cooper S-M, Feltrer Y, Calvi T, Strike T, Palmer C, Simcox S, Rodriguez P, Sanchez C, van Bolhuis H, Peck B, Eng J, Moittie S, Unwin S, Howatson G, Oxborough D, Stembridge MR, Shave RE (2020) Evaluation of relationships between results of electrocardiography and echocardiography in 341 chimpanzees (Pan troglodytes). Am J Vet Res 81:488–498.
- Drane AL, Calvi T, Feltrer Y, Curry BA, Tremblay JC, Milnes EL, Stöhr EJ, Howatson G, Oxborough D, Stembridge M, Shave R (2021) The influence of anesthesia with and without medetomidine on cardiac structure and function in sanctuary captive chimpanzees (Pan troglodytes). J Zoo Wildl Med 52:986–996.
- Erickson HH, Olsen SC (1985) Electrocardiogram, heart rate, and blood pressure in the chimpanzee. J Zoo Anim Med 16:89.
- Hirata S, Havercamp K, Yamanashi Y, Udono T (2022) Hepatitis C virus infection reduces the lifespan of chimpanzees used in biomedical research. Biol Lett 18:20220048
- Iwata I, Sakamoto T, Matsumoto T, Hirata S (2023) Noncontact measurement of heartbeat of humans and chimpanzees using millimeter- wave radar with topology method. IEEE Sens Lett. 7(11): 7006104
- Lonsdorf E, Travis D, Ssuna R, Lantz E, Wilson M, Gamble K, Terio K, Leendertz F, Ehlers B, Keele B, Hahn B, Gillespie T, Pond J, Raphael J, Collins A (2014) Field immobilization for treatment of an unknown illness in a wild chimpanzee (Pan troglodytes schweinfurthii) at Gombe National Park, Tanzania: Findings, challenges, and lessons learned. Primates 55:89–99.
- Olds JE, Goldacker A, Huneycutt D, Huneycutt SP, Taglialatela JP, Ward JL (2023) The AliveCor KardiaMobile ECG device allows electrocardiogram assessment in awake bonobos (Pan paniscus). Am J Vet Res.
- Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D (1992) An improved method for adjusting the QT interval for heart rate (the Framingham heart study). Am J Cardiol 70:797–801
- SerranoFinetti E, Hornero G, Mainar S, López F, Crailsheim D, Feliu O, Casas O (2023) A non-invasive, concealed electrocardiogram and bioimpedance measurement system for captive primates. J Exp Biol.
- Singh JP, Larson MG, O’Donnell CJ, Tsuji H, Evans JC, Levy D (1999) Heritability of heart rate variability: the Framingham heart study. Circulation 99:2251–2254
- Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, Dijk DJ (2007) Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res 16:148–155
- Wang D, Eckert J, Teague S, Al-Naji A, Haun D, Chahl J (2023) Estimating the cardiac signals of chimpanzees using a digital camera: validation and application of a novel non-invasive method for primate research. Behav Res Methods.

Fig. 1 Carrying out an electrocardiogram (ECG) on an adult male chimpanzee by using a portable device
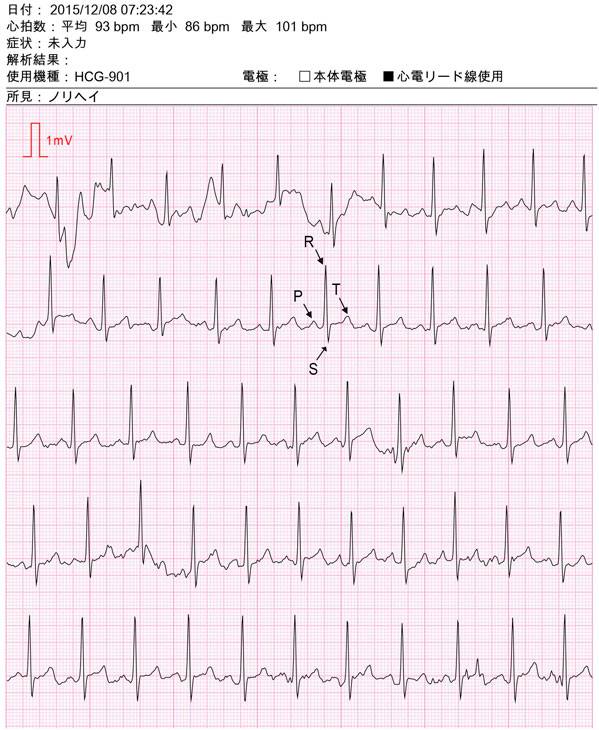
Fig. 2 An example of ECG recording data. The text in Japanese indicates the following: 1st line, “Date: 8 December 2015, at 07:23:42 am”; 2nd line, “Heart rate: mean 93 beats/min (bpm), min 86 bpm, max 101 bpm”; 3rd line, “Symptom: no input”; 4th line, “Analysis output: (none)”; 5th line, “Model used: HCG-901,” “Electrode: body electrode (unchecked), cable lead (checked)”; 6th line, “Note: Norihei (the name of the subject).” An example of a set of P, R, S and T waves are indicated for one heartbeat; Q waves are not clearly visible in this recording. A single continuous ECG recording is shown which is divided into five sections; the recording for only the first 35 s is shown; each section (from left to right) represents 7 s of the recording
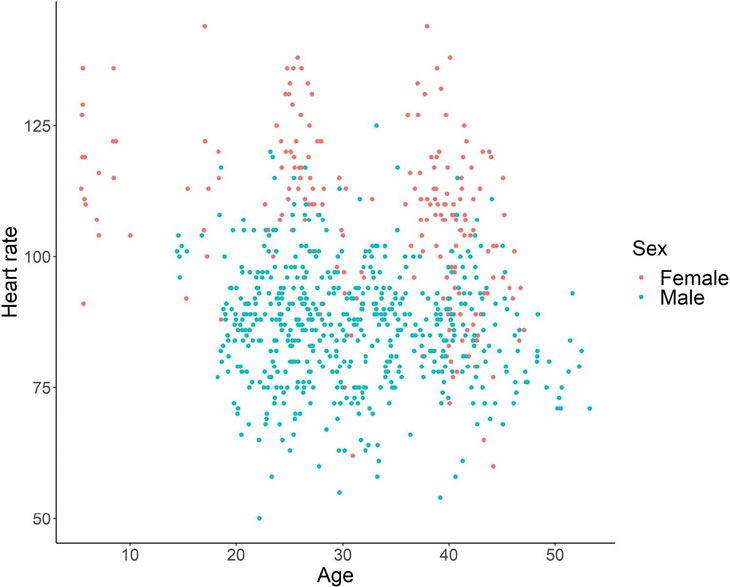
Fig. 3 Heart rate as a function of age
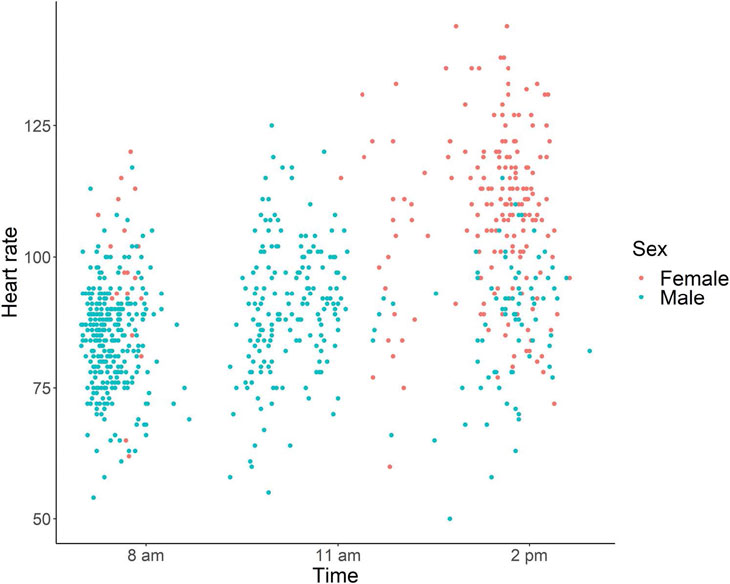
Fig. 4 Heart rate as a function of the time of day
n | Mean | SD | Minimum | Maximum | First quartile | Second quartile | Third quartile | |
|---|---|---|---|---|---|---|---|---|
Male | 22 | 86.5 | 11.1 | 50 | 125 | 79 | 87 | 93 |
Female | 10 | 106.4 | 16.1 | 60 | 144 | 96 | 108 | 117 |
Table 1: Summary of heart rate data from adult chimpanzees (≥ 14 years old)