Chimpanzee Down syndrome: a case study of trisomy 22 in a captive chimpanzee
Satoshi Hirata, Hirohisa Hirai, Etsuko Nogami, Naruki Morimura, Toshifumi Udono
DOI: 10.1007/s10329-017-0597-8Press Release
Our understanding of chromosome aberrations in humans can be significantly enhanced by studying similar pathologies in our closest evolutionary relatives.
Here, we report on the second ever confirmed case of chimpanzee chromosome aberration equivalent to human Down syndrome, this time at Kumamoto Sanctuary, Wildlife Research Center, Kyoto University. Developmental features that are characteristic of Down syndrome in humans were also observed in the chimpanzee, however, unlike the first reported case, this chimpanzee survived into adulthood.
We attempt to improve the quality of life of this chimpanzee, through providing and managing opportunities for normal social interaction. Such efforts are seen as key in caring for disabled chimpanzees in captivity.
Researchers document second case of “Down syndrome” in chimps - springer.comAbstract
We report a case of chimpanzee trisomy 22 in a captive-born female. Because chromosome 22 in great apes is homologous to human chromosome 21, the present case is analogous to human trisomy 21, also called Down syndrome. The chimpanzee in the present case experienced retarded growth; infantile cataract and vision problems, including nystagmus, strabismus, and keratoconus; congenital atrial septal defect; and hypodontia. All of these symptoms are common in human Down syndrome. This case was the second reported case of trisomy 22 in the chimpanzee. The chimpanzee in our case became blind by 7 years old, making social life with other chimpanzees difficult, but opportunities to interact with other conspecific individuals have been offered routinely. We believe that providing her with the best care over the course of her life will be essential.
Keywords
Chimpanzee, Trisomy, Chromosomal abnormality, Down syndrome, Cataract, Atrial septal defect
Introduction
Down syndrome in humans is a chromosome aberration caused by the presence of a third copy of chromosome 21 (HSA21), or trisomy 21 (Down 1866; Jacobs et al. 1959; Lejeune et al. 1959). Trisomy 21 is the most common chromosomal abnormality in humans, occurring in up to 1 in 600 live births, typically associated with retarded growth, cognitive delay, and physical disabilities (Antonarakis et al. 2004; Hernandez and Fisher 1996). McClure et al. (1969) reported the first case similar to Down syndrome in nonhuman animals. They described a female chimpanzee with trisomy 22. Her growth was retarded, and she had congenital heart disease. Two additional cases of trisomy 22 were later reported in two species of other great apes: gorilla and orangutan (Turleau et al. 1972; Andrle et al. 1979). In contrast to the normal diploid number of 46 in humans, the corresponding number of all of the great apes is 48, and chromosome 22 in great apes is homologous to HSA21 (Dutrillaux 1979; Jauch et al. 1992; Richard and Dutrillaux 1998; Ried et al. 1993). Features of Down syndrome in humans have been associated with band q22.3 of chromosome 21, and the hybridization site for this band was found on the equivalent ape chromosome 22 in chimpanzees, gorillas, and orangutans (Luke et al. 1995).
In this report, we describe a case of trisomy 22 in a captive-born female chimpanzee. The chimpanzee showed retarded growth and had infantile cataract, congenital heart disease, and hypodontia, features consistent with Down syndrome in humans.
Methods
A female chimpanzee named Kanako [Great Ape Information Network (GAIN) no. 480, see the GAIN website for more information: https://shigen.nig.ac.jp/gain/ViewIndividualDetail.do-id=400) was born on June 2, 1992, at a facility in Japan owned at the time by a private company. The facility was transferred to Kyoto University in 2011 and has been renamed Kumamoto Sanctuary, Wildlife Research Center, Kyoto University. The history and mission of the organization have been described in Morimura et al. (2011).
Kanako's mother was named Kanae and her father was named Tarou. Both Kanae and Tarou were wild-captured individuals from Sierra Leone. Kanae's year of birth was estimated to be 1979. Tarou's year of birth was estimated to be 1977. Kanako, Kanae, and Tarou all belonged to the western subspecies Pan troglodytes verus.
Kanako was delivered after an apparently uncomplicated pregnancy. Kanae had given birth to a male 24 months before Kanako was born. The father had a total of seven offspring prior to Kanako. Besides Kanako, all of the Kanae's and Tarou's offspring were healthy and apparently normal except for one of Tarou's offspring who was born premature and died at 7 days. Kanae was 13 years old and Tarou was 15 years old when Kanako was born. Thus, they were a relatively young mother and father when Kanako was conceived. Based on the last day of maximal swelling of the mother, we estimated the pregnancy period to be 230 days, which is within the normal range for a chimpanzee pregnancy. Body weight was measured routinely, and the data were compared with those obtained from other individuals housed at the same institute (see Hamada et al. 1996 for details).
The heart disease was diagnosed using an echocardiogram (General Electric LOGIQ iM) in 2014 during a routine physical examination when Kanako was 22 years old. Under sedation with ketamine hydrochloride (10 mg/kg), Kanako was put in a left lateral recumbent position, and a GE 3S sector transducer (1.5-3.6 MHz) was applied to the thoracic area. Before diagnosis, an electrocardiogram and a physical examination were conducted under sedation with ketamine hydrochloride (10 mg/kg) when she was 0, 1, 2, 4, 6, 7, 8, 13, and 18 years old, and a chest X-ray was taken when she was 2, 13, and 18 years old.
The echocardiogram results prompted us to conduct further chromosomal analysis. In 2015, when Kanako was 22 years old, 10 mL of venous blood was collected with a heparinized syringe. Leucocytes obtained by erythrocytes lysis treatment from 1 mL of the whole blood were cultured for 70 h to prepare metaphase chromosomes. The culture and chromosome preparations were conducted as previously described (Hirai et al. 1998, 2003). Metaphase spreads prepared on slide glasses were provided for fluorescence in situ hybridization (FISH) with human paint probe (HSA21, Qbiogene: Total-chromosome paint 21 probe-Green, France).
Results
Birth and growth
At one day of age, Kanako weighed 1940 g. The average weight for chimpanzee neonates is 1800 g (Gavan 1952). Staff noted that she was inactive, her arms and legs were limp, and she vocalized less frequently than other neonates in the same facility. When Kanako was 156 days old, her mother, Kanae, was anesthetized for a physical examination. As the anesthesia was wearing off, Kanae bit her own tongue. Kanako was then separated from Kanae for 4 days as Kanae recovered. When Kanako and her mother were reunited, the mother did not take care of Kanako. After that event, Kanako was hand-raised by human staff. During her first year she suffered from cough, snivels, fever, diarrhea, and swelling around her right eye, but such symptoms are not uncommon in young chimpanzees. Although systematic investigation of behavioral development was not conducted, there were no notable abnormalities recorded in the daily care-taking notes, other than the features described above, until vision problems appeared at around 1 year of age (see below). Hypotonia was not formally investigated. Hyperflexibility of the joints was not quantitatively measured, but the flexibility of the joints appeared to be larger than normal. No problems were noted with her locomotor movement.
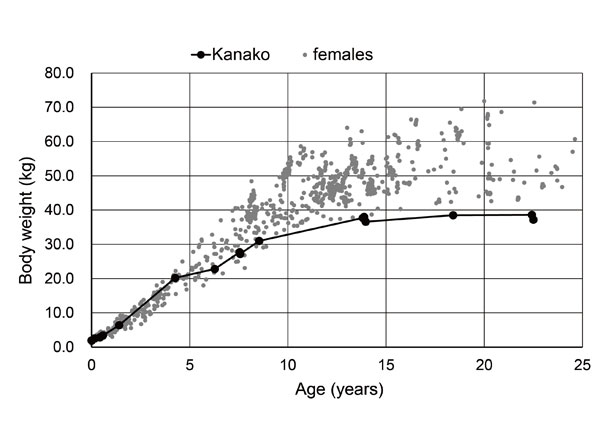
After age five, Kanako's growth was delayed compared to other individuals housed at the same facility (Fig. 1). In addition, she had hypodontia: only one maxillary premolar was present on each side and she did not have third molars.
Cataract and vision problem
At the age of 305 days, staff noticed leukocoria in Kanako's left eye. At the age of 352 days, leukocoria in her right eye was also noted. At the age of 354 days, staff observed that she searched for foods with her mouth, indicating clear decreased visual acuity. A funduscopy and slit-lamp examination confirmed the presence of cataracts.
At 2 years old, a cataract surgery was conducted for intraocular lens implantation for both eyes at the same time. However, Kanako repeatedly rubbed her eyes after the surgery, leading to postoperative inflammation. This inflammation caused pupillary block, which led to glaucoma and later glaucosis. Four months later, trabeculectomy was conducted. However, her glaucoma had advanced. Strabismus and nystagmus were also noted (Fig. 2). By age seven, her left eye showed corneal opacity and keratoconus. The eye might have been able to sense strong light because it moved when a light was shined on it. Staff repeatedly observed her fumbling and groping when she moved in a new environment or when she was searching for an object in front of her. Therefore, she was declared blind at 7 years of age. Her right eye had progressed to phthisis bulbi.

Atrial septal defect
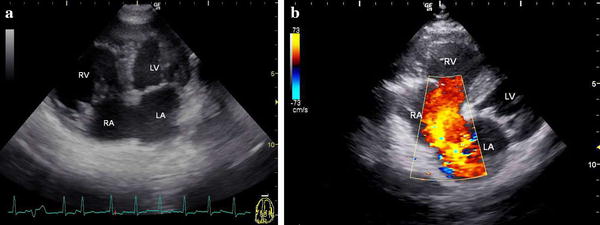
The echocardiogram with apical four-chamber view from the left thoracic wall revealed an atrial septal defect and right ventricular hypertrophy. Color Doppler imaging from the right parasternal area showed a large left-to-right shunt through the atrial septal defect (Fig. 3). Before detection of the atrial septal defect via echocardiogram, a cardiac murmur was not found. An electrocardiogram when Kanako was 18 years old revealed infrequent premature ventricular contractions. Enlargement of the right cardiac shadow was seen in a chest X-ray when she was 13 years old, but no clinical symptoms were detected.
Chromosome and blood analysis
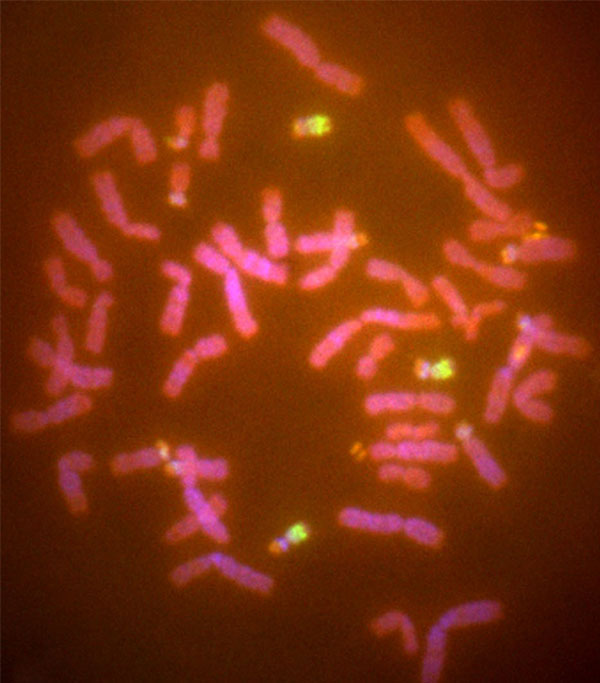
The results of the chromosomal analysis using FISH with HAS21 paint probes revealed that the metaphase spread of Kanako had a diploid chromosome number 49 (2n = 49) containing an extra chromosome. The extra chromosome was a member of three substances hybridized to HSA21 probes, being homologous to chromosome 22 of the chimpanzee. Kanako's karyotype was thus 49, XX, +22 (Fig. 4). Almost all hematological and serum chemical values were within normal range and are listed in Table 1. The values for albumin and chloride were slightly outside the normal range, possibly because of a difference in measurement system or a measurement error.
|
Item |
Kanako |
Average and range of normal chimpanzees (Howell et al. 2003) |
|
|---|---|---|---|
|
Erythrocytes |
(104/μL) |
549 |
510 (420-600) |
|
Hemoglobin |
(g/dL) |
14.0 |
13.6 (11.5-15.7) |
|
Hematocrit |
(%) |
46.4 |
42.0 (35.4-48.6) |
|
Thrombocytes |
(104/μL) |
11.4 |
23.0 (9.7-36.3) |
|
Leucocytes |
(/μL) |
13,600 |
9100 (2900-15,400) |
|
C-reactive protein |
(mg/dL) |
0.33 |
N/A |
|
Total protein |
(g/dL) |
7.6 |
7.5 (6.5-8.5) |
|
Albumin |
(g/dL) |
2.8 |
3.7 (3.0-4.5) |
|
A/G (albumin/globulin ratio) |
- |
0.6 |
1.0 (0.6-1.4) |
|
Total bilirubin |
(mg/dL) |
0.2 |
N/A |
|
ALP (alkaline phosphatase) |
(U/L) |
152 |
114.3 (33.0-269.8) |
|
AST (aspartate transaminase) |
(U/L) |
20 |
18.1 (5.1-31.2) |
|
ALT (alanine transaminase) |
(U/L) |
41 |
30.8 (10.5-51.1) |
|
LDH (lactate dehydrogenase) |
(U/L) |
269 |
320.8 (175.0-768.4) |
|
GGT (γ-glutamyltransferase) |
(U/L) |
30 |
28.5 (6.0-72.4) |
|
CK (creatine kinase) |
(U/L) |
121 |
229.0 (19.0-660.3) |
|
Cholinesterase |
(U/L) |
317 |
N/A |
|
Total cholesterol |
(mg/dL) |
214 |
212.2 (129.1-295.4) |
|
Triglycerides |
(mg/dL) |
65 |
109.2 (4.6-213.7) |
|
BUN (blood urea nitrogen) |
(mg/dL) |
11.3 |
11.5 (3.7-19.3) |
|
Creatinine |
(mg/dL) |
0.81 |
1.0 (0.4-2.2) |
|
Amylase |
(IU/L) |
93 |
N/A |
|
Glucose |
(mg/dL) |
69 |
83.6 (52.8-114.5) |
|
Sodium |
(mEq/L) |
136 |
138.4 (133.0-143.7) |
|
Potassium |
(mEq/L) |
3.8 |
3.8 (3.0-4.6) |
|
Chloride |
(mEq/L) |
90 |
101.1 (90.8-111.3) |
|
Calcium |
(mEq/L) |
9.1 |
9.1 (8.3-10.0) |
Social interaction
Because Kanako is blind, she cannot safely escape aggressive interactions and, therefore, cannot stay with other chimpanzees. Nevertheless, chimpanzees are social creatures, and for Kanako's quality of life, our goal was to provide an opportunity for her to stay together with a conspecific member. Because of her calm temperament, a wild-born female chimpanzee (named Roman) was selected to be an occasional partner of Kanako. Roman and Kanako were introduced in October 2010 when Kanako was 18 years old. They were initially in two adjacent rooms separated by bars in the introductory session. Six months later, after three introductory sessions, they were allowed to be in the same space (an outdoor enclosure or indoor room, depending on weather and other conditions). Since then, these encounters have occurred about once per month (1.2 times per month on average; Fig. 5). One session of their encounter lasts 30-60 min, with a staff member (EN) present to mediate their encounter. Roman was friendly to Kanako from the beginning of the introduction, and she occasionally tried to groom Kanako or invited her to play, but their interaction generally did not last long because Kanako did not move or react, or moved away. On some occasions, Kanako approached Roman and Roman gently touched her, but Kanako rarely touched Roman. They typically simply sat near each other and spent time quietly. At the beginning of the encounter session, Kanako almost always emitted a vocalization specific to her, which was a mixture of chimpanzee play grunt and food grunt, indicating her positive reaction toward the encounter session.

Discussion
This report describes a second case of chimpanzee trisomy 22 (the first was reported by McClure et al. 1969). Another case of a wild chimpanzee with abnormal behavioral development was reported by Matsumoto et al. (2016). The authors suspected Down syndrome, but chromosome abnormality was not tested. To the best of our knowledge, there is no other case where symptoms resembling Down syndrome have been noted in chimpanzees housed in Japan during the history of captive care. It is difficult to estimate the probability of a rare event using a small population, but given that around 500 chimpanzees have been born in captivity in Japan (Watanuki et al. 2014), the probability of this autosomal trisomy in chimpanzees may be comparable to that of trisomy 21 in humans, which occurs in up to 1 in 600 births (Hernandez and Fisher 1996). The chimpanzee reported in the present case experienced stunted growth, infantile cataracts, vision problems, congenital heart disease, and hypodontia. All of these symptoms are common in human Down syndrome (Down 1866; Bull 2011). The present case, along with the previously reported cases in apes, confirms that trisomy of great ape chromosome 22 results in a disorder similar to human Down syndrome (McClure et al. 1969; Turleau et al. 1972; Andrle et al. 1979).
In the first reported case of chimpanzee trisomy 22, researchers evaluated behavioral development in the affected chimpanzee and showed that development of sitting and standing postures were delayed (McClure et al. 1969). Conclusions about retardation of behavioral development cannot be made in Kanako's case because systematic investigation in this regard was not conducted. Furthermore, data for retrospective assessment, such as video recordings, are not available. However, the lack of abnormalities noted in daily care-taking before the age of one, except for neonatal inactivity and limp limbs, suggests that there was no severe retardation in behavioral development. Kanako's infantile cataracts that began to emerge at around 1 year of age and her eventual blindness prevented us from evaluating her behavioral development afterwards, because behavioral abnormalities are difficult to distinguish from visual problems. The trisomic chimpanzee reported by McClure et al. (1969) died before reaching 2 years of age. Kanako has survived until adulthood and is alive at the time of writing the present report. Our goal has been to provide Kanako with the best care and quality of life. One critical component of this effort is giving her an opportunity to interact with another chimpanzee (see Miyabe-Nishiwaki et al. 2010, Hayashi et al. 2013, and Sakuraba et al. 2016 for another case of care of a disabled chimpanzee; see also Matsuzawa 2016). A detailed and thorough pathological examination of Kanako, including autopsy imaging, will be conducted after her natural term.
Acknowledgements
We have complied with the ethical standards in the treatment of the chimpanzees with the guidelines of the Primate Society of Japan. We thank the staff at Kumamoto Sanctuary for support in caring for the chimpanzees. The care of the chimpanzees and the present study was financially supported by JSPS grant #23220006, 26245069, 25119008, 242550099, 15H05709, 16H06301, 16H06283, JSPS-LGP-U04, JSPS core-to-core CCSN.
References
- Andrle M, Fiedler W, Rett A, Ambros P, Schweizer D (1979) A case of trisomy 22 in Pongo pygmaeus. Cytogenet Genome Res 24:1-6
- Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S (2004) Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Gen 5:725-738
- Bull MJ (2011) Health supervision for children with Down syndrome. Pediatrics 128:393-406
- Down JLH (1866) Observations on an ethnic classification of idiots. Lond Hosp Rep 3:259-262
- Dutrillaux B (1979) Chromosomal evolution in primates: tentative phylogeny from Microcebus murinus (Prosimian) to man. Human Genet 48:251-314
- Gavan JA (1952) Birth order and birth weight in the chimpanzee. Am J Phys Anthropol 10:23-30
- Hamada Y, Udono T, Teramoto M, Sugawara T (1996) The growth pattern of chimpanzees: somatic growth and reproductive maturation in Pan troglodytes. Primates 37:279-295
- Hayashi M, Sakuraba Y, Watanabe S, Kaneko A, Matsuzawa T (2013) Behavioral recovery from tetraparesis in a captive chimpanzee. Primates 54:237-243
- Hernandez D, Fisher EM (1996) Down syndrome genetics: unravelling a multifactorial disorder. Hum Mol Gen 5:1411-1416
- Hirai H, Hasegawa Y, Kawamoto Y, Tokita E (1998) Tandem duplication of nucleolus organizer region (NOR) in the Japanese macaque, Macaca fuscata fuscata. Chromosome Res 6:191-197
- Hirai H, Mootnick AR, Takenaka O, Suryobroto B, Mouri T, Kamanaka Y, Katoh A, Kimura N, Katoh A, Maeda N (2003) Genetic mechanism and property of a whole-arm translocation (WAT) between chromosomes 8 and 9 of agile gibbons (Hylobates agilis). Chromosome Res 11:37-50
- Howell S, Hoffman K, Bartel L, Schwandt M, Morris J, Fritz J (2003) Normal hematologic and serum clinical chemistry values for captive chimpanzees (Pan troglodytes). Comp Med 53:413-423
- Jacobs P, Brown WC, Baikie AG, Strong JA (1959) The somatic chromosomes in mongolism. Lancet 273:710
- Jauch A, Wienberg J, Stanyon R, Arnold N, Tofanelli S, Ishida T, Cremer T (1992) Reconstruction of genomic rearrangements in great apes and gibbons by chromosome painting. Proc Natl Acad Sci USA 89:8611-8615
- Lejeune J, Gautier M, Turpin R (1959) Etude des chromosomes somatiques de neuf enfants mongoliens. Compte Rendu d'Acad Sci 248:1721-1722
- Luke S, Gandhi S, Verma RS (1995) Conservation of the Down syndrome critical region in humans and great apes. Gene 161:283-285
- Matsumoto T, Itoh N, Inoue S, Nakamura M (2016) An observation of a severely disabled infant chimpanzee in the wild and her interactions with her mother. Primates 57:3-7
- Matsuzawa T (2016) Euthanasia is not an option: 10 years' care of a chimpanzee with acute tetraparesis. Primates 57:291-293
- McClure HM, Belden KH, Pieper WA, Jacobson CB (1969) Autosomal trisomy in a chimpanzee: resemblance to Down's syndrome. Science 165:1010-1012
- Miyabe-Nishiwaki T, Kaneko A, Nishiwaki K, Watanabe A, Watanabe S, Maeda N, Kumazaki K, Morimoto M, Hirokawa R, Suzuki J, Ito Y, Hayashi M, Tanaka M, Tomonaga M, Matsuzawa T (2010) Tetraparesis resembling acute transverse myelitis in a captive chimpanzee (Pan troglodytes): long-term care and recovery. J Med Primatol 39:336-346
- Morimura N, Gen'ichi I, Matsuzawa T (2011) The first chimpanzee sanctuary in Japan: an attempt to care for the “surplus" of biomedical research. Am J Primatol 73:226-232
- Richard F, Dutrillaux B (1998) Origin of human chromosome 21 and its consequences: a 50-million-year-old story. Chromosome Res 6:263-268
- Ried T, Arnold N, Ward DC, Wienberg J (1993) Comparative high-resolution mapping of human and primate chromosomes by fluorescence in situ hybridization. Genomics 18:381-386
- Sakuraba Y, Tomonaga M, Hayashi M (2016) A new method of walking rehabilitation using cognitive tasks in an adult chimpanzee (Pan troglodytes) with a disability: a case study. Primates 57:403-412.
- Turleau C, de Grouchy J, Klein M (1972) Phylogenie chromosomique de I'homme et des primates hominiens (Pan troglodytes, Gorilla gorilla, et Pongo pygmaeus): essai dereconstitution du caryotypes de I'ancestre commun. Ann Genet 15:225-240
- Watanuki K, Ochiai T, Hirata S, Morimura N, Tomonaga M, Idani G, Matsuzawa T (2014) Review and long-term survey of the status of captive chimpanzees in Japan in 1926-2013. (in Japanese with English summary). Primate Res 30:147-156