Humans often have a better memory of emotional events than neutral events. From the
comparative-cognitive perspective, we explored the enhancement of recognition memory by emotion in
chimpanzees (Pan troglodytes) using a serial probe recognition task. In this task, we sequentially
presented a list of pictures to subjects and then tested their recognition of specific pictures from
within the list. We selected pictures of aggressive chimpanzees as emotional stimuli and less
tensed, relaxed chimpanzees as neutral stimuli. In Experiment 1, we gave four-item lists to two
young chimpanzees; one showed significantly greater recognition of pictures depicting aggressive
chimpanzees than neutral ones. In Experiment 2, this chimpanzee was further tested using a
recognition task with eight-item lists. The subject again showed better recognition of emotional
stimuli than neutral. Furthermore, the presence of an emotional stimulus in the list also
facilitated recognition of the neutral item immediately following it. Overall, although only one of
the two chimpanzees showed enhanced recognition memory by emotional stimuli, this is the first
demonstration of such a response in the chimpanzee. The findings are discussed in comparison with
those of human studies.
Introduction
Emotion interacts with and influences other domains of cognition. In humans, the
cognitive domain in which the influence of emotion is best understood is memory (Dolan 2002).
Studies have shown that emotionally arousing events are more likely to be recollected than
neutral events (e.g., Christianson 1992b). Recent findings from neuroimaging,
neuropsychological, drug, and neural stimulation studies indicate that emotional stimuli engage
specific cognitive and neural mechanisms that enhance memory (Cahill et al. 1994; Cahill and
McGaugh 1998; Hamann 2001). From an evolutionary perspective, such “emotional memory” is likely
to have both immediate and future relevance to survival and reproductive success (Hamann 2001)
because emotional arousal signals important occasions such as fights among conspecifics,
encounters with predators, finding favourite foods, and mating. Therefore, certain
characteristics of emotional information could be perceived and retained in a way common to
humans and non-human animals. As a candidate for a common mechanism, Zajonc (1980) proposed that
affective reactions to stimuli are the first reactions that influence cognitive processes such
as recognition memory and feature discrimination. Similarly, in an extensive review of emotional
memory, Christianson (1992a) suggested that emotion-eliciting stimuli undergo some preferential
processing mediated by factors related to early perceptual processing and late conceptual
processing. Comparative studies of non-human primates may be useful for exploring the
phylogenetic origins of the link between memory and emotion. A number of studies have shown that
primates store detailed memories that contribute to survival or reproductive success (e.g.,
Menzel 1973; Dittrich 1994). However, few studies have taken a direct approach to the
interaction of emotion and memory in primates. Thus, we examined such an interaction in the
chimpanzees, the closest relatives of humans.
Extensive studies of animal memory have revealed that basic features of animal
memory are similar to those of human memory (Kendrick et al. 1986), although comparing memories
between different species is somewhat difficult. Buchanan et al. (1981) trained a chimpanzee in
a multiple-item recognition task and obtained similar list-memory performance to that seen in
humans. Sands and Wright (1980) tested memory in a rhesus macaque using a serial probe
recognition task. A list of pictures was presented to the macaque, and after a short delay, two
probe items were presented. One of the items was from the list just presented, while the other
was not. The serial position curve obtained from the macaque was similar to that for a human
participant. The serial probe recognition task is often used in animal memory studies (dolphins,
Thompson and Herman 1977; pigeons and macaques, Wright et al. 1985). In the present study, we
used this type of task to test chimpanzees’ recognition memory of emotionally arousing pictures
from lists.
In human studies, the injury or death of a character or a crime in the story is
often used for the emotional stimuli (Christianson 1992a). For chimpanzees, foods, mating, and
aggressive behaviors of conspecifics are thought to be emotional scenes. In an experimental
setting, non-linguistic methods can be designed using picture stimuli. For example, some studies
have reported physiological changes in chimpanzees viewing the pictures that have emotional
contents (Parr and Hopkins 2000; Parr 2001). In addition, Parr (2001) demonstrated that the
chimpanzee participants used still pictures of chimpanzee facial expressions to categorize
emotional video scenes according to their positive and negative valance (e.g., matching a
hypodermic needle to the bared-teeth display), suggesting their emotional awareness. In the
present study, we used still pictures of chimpanzee aggressive behaviors for emotional stimuli
(see “Methods” of Experiment 1 for details).
We conducted two experiments. In the first, we initially trained chimpanzees on a
simple delayed matching task and then shifted to a task with four-item lists using
non-chimpanzee stimuli to observe the basic characteristics of list memory in chimpanzees. After
these pretraining phases, we ran test sessions in which we presented lists containing neutral
and emotional pictures and those containing only neutral pictures. If emotional contents enhance
chimpanzee memory, it is predicted that emotional pictures would be better recognized than
control pictures. In humans, emotional items (or events) can affect other temporally proximate
items or events. However, no studies to date have examined these effects on non-human primates.
To examine the effects of emotional items on adjacent neutral items, we additionally tested one
of the two chimpanzee participants using eight-item lists in Experiment 2.
Experiment 1
Participants
Two young chimpanzees (Pan troglodytes; Pal and Cleo, both female)
participated in Experiment 1. Both were 6 years old at the onset of the experiment. They had
experience taking part in various types of testing for cognitive development (Tanaka et al. 2003;
Tomonaga et al. 2003, 2004; Matsuzawa et al. 2006; Tomonaga 2007) including computer-controlled
perceptual–cognitive tasks (Tanaka et al. 2003; Tanaka 2007a, b). They had also been trained
on matching-to-sample tasks but had never experienced experimental tasks in which emotional
responses were rewarded. Both had been reared by their own biological mothers from birth and lived
in a social group of 14 individuals in an environmentally enriched outdoor compound and an attached
indoor residence. No special food or water deprivation was conducted during the study period. Care
and use of the chimpanzees adhered to the 2002 version of the Guideline for Care and Use of
Laboratory Primates by the Primate Research Institute, Kyoto University. The experimental protocol
was approved by the Animal Welfare and Care Committee of the institute.
Apparatus
The experimental sessions were conducted in an experimental booth (1.6m
W×1.8m
D×2.1m
H). Two sets of 15-inch LCD monitors with touch-sensitive screens (1,028 × 768 pixels,
32 bit color, Touch panel systems, U2-BA; Attic, TP-153U) were installed on different sides of the
booth. Touch to the stimulus on the screen was defined as a response. A universal feeder (Biomedica,
BUF-310) delivered pieces of food (apples or raisins) into a food tray below the monitor. All the
equipments were controlled by a personal computer running a program written in Microsoft Visual
Basic 6.0.
Stimuli
We prepared 200 full-color still pictures of fish, reptiles, birds, and sea mammals (10.0 × 5.6 cm
on the screen; see Fig. 1) for the initial training phases (referred to as
“non-chimpanzee stimuli”). For the test stimuli, we prepared still color pictures of wild
chimpanzees (10.0 × 6.6 cm) from video recorded in Bossou, Guinea, West Africa, for research
purposes (Biro et al. 2003). We used Adobe Photoshop 6.0 to prepare the images.
 Fig. 1
Fig. 1 Examples of aggressive and neutral stimuli used in the test sessions.
Chimpanzee photographs courtesy of Tetsuro Matsuzawa
Pictures of chimpanzees were categorized into two types based on their behaviors. The “Aggressive”
(A) category contained images of aggressive behaviors performed by chimpanzee(s), while the
“Neutral” (N) category contained images of less tensed behavior (see Fig. 1).
Aggressive behaviors were defined as attack, defence, and threat (Goodall 1986). Previous studies
have reported that chimpanzees responded emotionally to pictures of chimpanzee aggressive behaviors
(Parr and Hopkins 2000; Parr 2001). We prepared 76 pictures for each category. From among these
pictures, we selected the best 40 within each category based on the rating scores of subjective
emotional value and picture quality, as judged independently by three researchers with extensive
experience observing chimpanzees.
Procedure
Both participants came to the experimental booth with their mothers but were trained separately
with different monitors in the booth. Almost no interaction occurred between them during the
task. Each trial began with the presentation of a warning stimulus at a random position on the
screen. When the chimpanzee touched the stimulus, the first item of the list was presented as a
sample stimulus for 1 s before being masked with a gray square. Touching the mask was followed
by the presentation of the next item with a 0.4-s delay at a position different from the
previous item. After all the sample items were thus presented, probe items were displayed after
a 1.2-s delay. One of the probe items was chosen from the pictures in the list (correct item)
while the other was not part of the list (incorrect item). Incorrect items were selected in a
pseudorandom order from among the unused pictures in the given trial. Response to the correct
item was rewarded with a piece of apple and a chime, while an incorrect response was not
rewarded and was followed by a buzzer sound.
The training phase began with a simple matching task (i.e., single-item lists). When accuracy
in two consecutive sessions exceeded 80%, we introduced two-item lists to the participants.
Sample items were thus added successively until the list length reached four items. Each
training session consisted of 36 trials, and the chimpanzees were given two to three sessions
daily. The training pictures were presented in a session-based trial-unique manner; that is, the
same picture was never presented more than once within the same session. The order of test
pictures in each sample list was changed after each session.
After reaching the criterion for the four-item lists, we conducted additional sessions of
steady-state training with four-item lists to obtain serial position curves and then moved on to
test sessions. In Experiment 1, we prepared two control conditions, N-N-N-N (all-N lists) and
A-A-A-A (all-A lists), in which N (neutral) and A (aggressive) denote the category of pictures
for each position of the four-item list, and four test conditions, in which one of the list
items (called the test item) was in a different category from the other list items: N-A-N-N and
N-N-A-N (A-with-Ns lists) and A-N-A-A and A-A-N-A (N-with-As lists). The latter two
counterbalanced conditions (lists with one emotional and three neutral pictures and those with
one neutral and three emotional pictures) were controls to exclude the possibility of better
recognition of emotional pictures simply because they were in a different category than neutral
pictures (Jitsumori et al. 1989; Strange et al. 2003). A test item appeared only in the second
or third position to separate the effects of emotional items from serial position effects such
as primacy or recency effects (i.e., superior performance on last and first items relative to
medial list items). Thus, although all list items were used as the probe items, only the second
and third list items were analyzed. The accuracy for each test item was compared to that for the
control item at the same list position. Each condition was pseudorandomly presented in a
session. Each test session consisted of 32 trials, and each block consisted of five sessions.
Test pictures were presented so that each picture was seen once before any repetition. The order
of test pictures in each sample list was changed after all the pictures were presented. Both of
the probe items were in the same category to ensure that chimpanzees did not discriminate them
based on category difference. Each participant was given eight session blocks (i.e., 40
sessions). We also collected the response-time data for correct trials.
Results
Training phase
Pal reached the criterion in 8, 8, 4, and 2 sessions and Cleo in 6, 21, 9, and 11 sessions for
training phases involving list lengths of 1 through 4 items, respectively. As stated in the “Methods”,
we continued training with four-item lists until both the participants had completed 18 sessions. We
then calculated the mean accuracy for each list position in the 18 sessions. Figure 2
illustrates the upward curves for the first through last list items for both the participants.
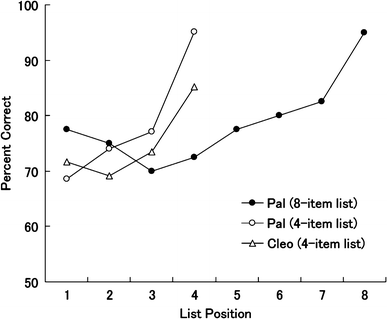 Fig. 2
Fig. 2 The effect of list position on recognition memory of the non-chimpanzee
items for both the subjects.
Open symbols show the results from Experiment 1, and the
filled circles show the results from Experiment 2
Test conditions
Overall, Pal and Cleo were, respectively, 76.8% (SEM ± 1.51) and 71.8% (SEM ± 1.65) correct in the
test session blocks. For each test session block, we calculated the mean accuracy of each list
position under each list condition. To test the effects of the emotional stimuli on recognition
memory, we compared the mean accuracy for test items at the second and/or third positions for
A-with-Ns and N-with-As lists and that for control items at the corresponding positions for all-N
and all-A lists. Figure 3 shows the mean accuracy for the test and corresponding
control items for each participant. As shown in these figures, one of the two chimpanzees, Pal,
exhibited better recognition for emotional pictures among neutral ones than for neutral items at the
corresponding list position (87.5% correct vs. 67.5% correct, paired-comparison t-test,
t(7) = 4.32, P = 0.003, using Bonferroni’s corrections for the two
comparisons of test-control pairs; the corrected alpha level for statistical significance was set to
0.025, which corresponds to 0.05). However, recognition accuracy was not facilitated when neutral
items were presented among emotional ones (78.8 vs. 74.4%, t(7) = 1.21, P
= 0.26). In contrast to Pal, the other participant, Cleo, did not exhibit enhanced recognition
neither under the all-N and N-with-As list conditions (70.0 vs. 73.7%, t(7) = 0.426,
P = 0.68), nor under the all-A and A-with-Ns list conditions (71.8 vs. 76.2%,
t(7) = 0.747, P = 0.48).
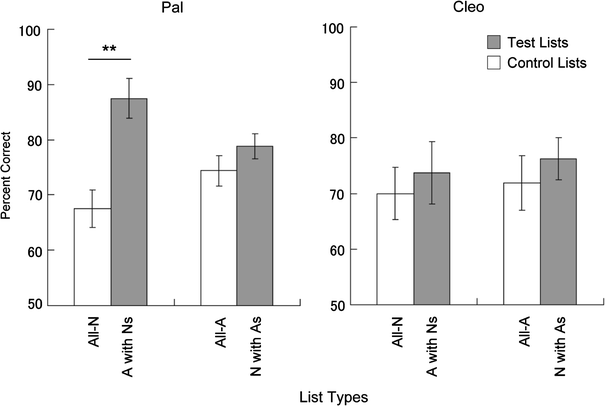 Fig. 3
Fig. 3 Percentage of correct trials (±SEM) for control and test lists.
A picture of aggressive chimpanzee behaviours,
N picture of less tensed,
relaxed chimpanzee behaviours. **
P < 0.01
To further investigate the individual differences between the two chimpanzees, we calculated the
mean response times for the last training session and the first test session for each participant.
Response times did not differ between chimpanzees in the last training session [t-test,
1,055 ± 38 (SEM) ms for Pal vs. 1,136 ± 56 ms for Cleo, t(36) = 1.18, P =
0.23], but Pal showed significantly longer response times than Cleo for the first test session
(2,299 ± 192 ms vs. 1,499 ± 99 ms, t(32) = 3.63, P < 0.001 using
Bonferroni’s correction for the two comparisons; the corrected alpha level was set to 0.025).
Discussion
Both the young chimpanzees successfully mastered the serial probe recognition task and showed
upward serial position curves from the first through the last items. Similar serial position curves
have been reported for both non-human animals (Thompson and Herman 1977; Morimura and Matsuzawa
2001) and humans (Hines 1975).
One of the chimpanzees, Pal, exhibited significant enhancement in the recognition memory of
emotional items. Because the neutral and emotional stimuli were presented with equal frequency, we
can exclude training bias for each category. Pal only showed enhanced recognition when the emotional
items were embedded among neutral items in the list, but not vice versa. This asymmetry avoids the
simple category–contrast account that predicts the symmetrical effect of enhanced recognition
for an odd item among items from another category.
By contrast, the other participant, Cleo, showed no enhancement of recognition memory. Several
possible explanations exist for these individual differences. The most plausible is that the
“emotional” pictures were not sufficiently salient for Cleo, possibly because of the size and
clarity of the images, the appropriateness of the scenes, or Cleo’s task-solving strategy.
Behavioral observations provided indirect but suggestive evidence for differences between the
individuals. For example, we observed on several occasions that Pal exhibited emotional responses
when she was shown emotional stimuli; she intensively attended to the stimulus, erected her hair,
shook her body, and sometimes hit the pictures. We did not observe such emotional responses in Cleo.
Pal’s mean response time for the last training session was almost the same as Cleo’s but was
significantly slower for the first test session. In the first test session, Pal held her response to
the probe items and attended to them longer than did Cleo. It is therefore possible that Cleo did
not pay enough attention to the stimuli or to the task to figure out the contents of the stimuli.
The stimuli were still pictures; thus, some concentration was probably required to discern the
meanings. It is also plausible that Cleo paid attention mainly to local features, whereas Pal
focused on the emotional contents in the picture.
These individual differences should be tested in future studies under different settings. In
addition, greater study control is necessary to avoid the category–contrast account because
the observed enhancement might reflect the asymmetry of the visual properties of the stimulus sets,
rather than their emotional contents.
Overall, although only one of the two chimpanzees showed enhanced recognition memory of emotional
items, this is the first demonstration of such a response in the chimpanzee. Experiment 2 further
tested Pal to replicate the results of Experiment 1 in a task with eight-item lists. By increasing
the number of items, we were able to further examine how emotional items affected memory of other
adjacent items.
Experiment 2
Participant, apparatus, and stimuli
Pal, the chimpanzee that showed enhanced recognition of emotional items in Experiment 1,
participated in this experiment. The experimental setting was identical to that of the previous
tests. Training stimuli were the same as those in Experiment 1. For test stimuli, we selected new
set of 20 pictures in the emotional category and 60 pictures in the neutral category from the
stimuli pool used in Experiment 1.
Procedure
The flow of each trial was identical to that in Experiment 1. Pal was shifted to this experiment
immediately after completing Experiment 1. As in Experiment 1, she was trained with non-chimpanzee
stimuli to extend the list length. The list length was increased by one as soon as Pal showed
accuracy better than 75% correct for two consecutive sessions, until eight-item lists were reached.
Each training session consisted of 18–24 trials depending on the list length.
In the test condition, Pal was given 8 five-session blocks. Each session consisted of 24 trials, in
which 16 trials used test stimuli and the other 8 trials used non-chimpanzee stimuli. We prepared
two types of list conditions, all-N lists (N-N-N-N-N-N-N-N) as the control and A-with-Ns lists
(N-N-N-A-N-N-N-N and N-N-N-N-A-N-N-N) as tests. Emotional items appeared either in the fourth or
fifth position. The two types of lists appeared in equal number but were distributed randomly within
a session.
Results and discussion
Pal was given five, two, two, and five sessions to reach the criterion for training with list
lengths of five, six, seven, and eight, respectively. To obtain serial position curves, we used the
non-chimpanzee stimuli presented in the test sessions. We calculated the mean accuracy for each list
position of the non-chimpanzee stimuli. There was an upward curve from the first through the last
list items (Fig. 2), as in Experiment 1.
Overall, Pal was 72.7% (SEM ± 2.07) correct in the test session blocks. For each test session
block, we calculated the mean accuracy of each list position under each list condition. Because
emotional items appeared either at the fourth or fifth position in A-with-Ns lists, the comparison
of A-with-Ns and All-N lists was based on relative list positions: A-with-Ns list items at A-2, A-1,
A, A + 1, and A + 2 positions were compared with all-N list items at the corresponding positions (A:
emotional items; A-2, A-1: 2 items, 1 item before emotional items, respectively; A + 1, A + 2: 1
item, 2 items after emotional items, respectively). Pal’s correct responses for A-with-Ns list items
at A-2 through A + 2 positions were 52.5, 70, 92.5, 85 and 65% (SEM = 10.52, 8.23, 9.58, 4.53, and
3.77, respectively), and her correct responses for All-N list items at corresponding positions were
65, 65, 57.5, 62.5 and 70% (SEM = 9.95, 8.45, 3.65, 5.0 and 5.0, respectively).
To visualize the comparison simply, we calculated the differences in accuracy between A-with-Ns
condition and All-N condition; from the correct responses of A-with-Ns list items at A-2 through A +
2 positions, All-N list items at corresponding positions are subtracted. Figure 4 shows
the results, with the vertical axis indicating the difference in accuracy (percent correct) between
emotional and neutral items, and the horizontal axis showing the relative list position. Pal
exhibited better accuracy for emotional items and the neutral item immediately following an
emotional item in the test lists than for neutral items at the corresponding position in the control
lists (Fig. 4).
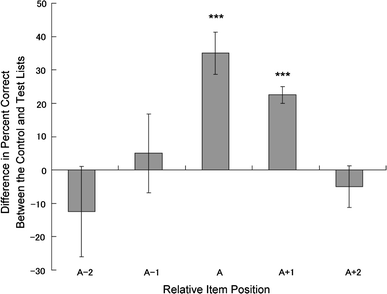 Fig. 4
Fig. 4 Differences in accuracy between test and control lists as a function of
relative list position. Positive scores illustrate better recognition for emotional items, whereas
negative scores indicate better recognition for neutral items.
Error bars show the
standard error of the mean (SEM). ***
P < 0.001
Two-way (list position [5] × list condition [2]) repeated-measures analysis of variance verified
these results. Pal showed better performance on test trials than on control trials [73 vs. 64%,
F(1,7) = 5.72, P = 0.048, ηp
2 = 0.45]; the interaction was also significant [F(4, 28) = 4.53,
P = 0.006, ηp
2 = 0.90]. Post hoc analyses of simple effects revealed significant differences in
accuracy at position A [F(1,7) = 31.18, P = 0.001, ηp
2 = 0.81] and A + 1 [F(1, 7) = 81.0, P < 0.001,
ηp
2 = 0.92 ].
As in the four-item list condition of Experiment 1, Pal again showed enhanced recognition memory of
emotional items. In addition, her performance on the recognition of items immediately following the
emotional item was improved. We have two possible explanations for this effect. First, Pal could
have been more motivated to inspect the emotional items and, as a consequence, paid more attention
to the subsequent items than to the controls. Focused attention to both emotional items and
subsequent items could have led to their enhanced recognition. It should be noted that the emotional
stimuli were pictures of chimpanzee aggressive behaviors, which tend to induce strong and sustained
orienting responses in chimpanzees. Daily observations clearly show that aggressive behaviors in
chimpanzees capture conspecifics’ focused attention. While the first explanation refers to an
encoding process, the second explanation refers to a post-encoding process, “consolidation” by which
new memories become more permanent and resistant to loss. Studies have pointed out that a pattern
perceived under high arousal shows stronger permanent memory, as the gradual process of
consolidation proceeds (e.g., Kleinsmith and Kaplan 1963; Hamann 2001). Pal’s performance decreased
gradually from the emotional items to the subsequent neutral items. This could be explained by a
decreasing arousal level. However, as a shortcoming of this explanation, the effect of arousal on
consolidation is thought to work slowly over time (Kleinsmith and Kaplan 1963; Cahill and McGaugh
1998), while the recognition memory was tested immediately after presenting sample items in this
study. We compare the findings with those of human studies in the general discussion that follows.
General discussion
We examined the emotional memory in chimpanzee subjects using a serial probe recognition task. A
list of pictures that depicted aggressive and less tensed, relaxed chimpanzees was presented
sequentially, followed by testing for the recognition memory of pictures contained within the list.
We observed enhanced recognition of emotional stimuli in one of the subjects (Experiments 1 and 2)
and also of neutral items immediately following emotional items in the same subject (Experiment 2).
It is worth comparing these results with the phenomena and mechanisms of emotional memory in human
studies, irrespective of some differences in the experimental settings. Many studies have
demonstrated that emotional events/stimuli tend to be well remembered (Kleinsmith and Kaplan 1963;
Heuer and Reisberg 1990; Christianson 1992b) In contrast, conflicting phenomena also exist. Clinical
observations have found that patients sometimes repress unpleasant memories by keeping them out of
their awareness (Goodman et al. 2003). In addition, several laboratory studies have shown that the
details of emotionally arousing events/stimuli are remembered poorly (Loftus and Burns 1982; Strange
et al. 2003). A deteriorated memory of events that occurred prior to an emotional event is called
emotion-induced retrograde amnesia. Overall, despite extensive research on emotional memory, human
studies have shown mixed results (see Christianson 1992a for a review).
The chimpanzee generally showed enhanced recognition memory of the stimuli, and not deteriorated
recognition. How can we interpret these results with the mixed reports for humans? One explanation
is that our stimuli evoked the types of arousal associated with orienting responses that enhanced
the recognition memory of the stimuli. Orienting responses are typical behaviors of chimpanzees when
viewing aggressive behavior of conspecifics. In humans, similar results and interpretations were
made by Heuer and Reisberg (1990), who pointed out that the different types of arousal might lead to
different results. If a type of arousal is too painful, it might prevent the recollection of the
stimuli/events and deteriorate the memory of them. Also, some types of arousal such as flight might
narrow the attention to the stimuli/events and deteriorate the memorization of their details.
Although we have demonstrated enhanced recognition memory of emotional stimuli in the chimpanzee,
it is too early to conclude that chimpanzee emotional memory is similar to that of humans. Further
experimentation is needed to confirm and expand on our findings.
First, the number of participants should be increased to confirm the robustness of our findings.
Second, to exclude category–contrast effects from the emotional enhancement of memory, new
stimulus sets should be introduced for controls. For example, simpler stimuli such as chimpanzee
facial expressions may weaken the asymmetric visual properties of the stimulus sets and highlight
the differences in emotional content. Third, to explain the observed individual differences, the
stimulus sets must be improved from the perspective of image quality and emotional saliency. For
example, more realistic stimuli such as movie clips might cause more individuals to show enhanced
recognition of emotional stimuli. In addition, to expand this finding of a serial probe recognition
task to other types of tasks, different procedures should be introduced. For example, using a
preferential looking paradigm, we predict that looking time will show an interaction between
familiarity and the emotional content of the stimulus sets.
We used images of aggressive behavior as emotional stimuli. It may be worth doing the same
experiment using other types of emotionally arousing images such as those of pant-hoot or play
activities or of foods. In addition, we examined the short-term retention of emotional stimuli. The
effect of long-term retention on memory has not yet been examined. Furthermore, in terms of age
differences, in humans, older adults have been found to have less enhanced memory than younger
adults, especially for negative emotional stimuli/events (Charles et al. 2003). This is thought to
be because of enhanced emotion regulation in older adults. Our chimpanzee participants were 6 years
old at the onset of the experiments. It would be valuable to examine the effect of age on emotional
memory in terms of emotion regulation in chimpanzees.
Acknowledgments
This study was conducted as a part of bachelor’s degree work by the first author. The study and the
preparation of the manuscript were financially supported by the Japan Society for the Promotion of
Science (JSPS)/Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan,
Grants-in-Aid for Scientific Research (#16002001, 16300084, and 19300091). We thank Dr. Tetsuro
Matsuzawa for his advice throughout the study and for kindly allowing us to use video footage of
chimpanzees in Bossou as stimuli. We also thank Suzuka Hori for her help during the experiments and
Dr. D. Biro for her reading of and comments on earlier drafts. Thanks are also due to the members of
the Human Evolution Laboratory of Kyoto University for their valuable comments and the staff of the
Language and Intelligence Section and the Center for Human Evolution Modeling Research at the
Primate Research Institute for their care of the chimpanzees.
References
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T (2003) Cultural
innovation and transmission of tool use in wild chimpanzees: evidence from field experiments.
Anim Cogn 6:213–223
- Buchanan JP, Gill TV, Braggio JT (1981) Serial position and clustering effects in a chimpanzee’s
“free recall”. Mem Cognit 9:651–660
- Cahill L, McGaugh JL (1998) Mechanisms of emotional arousal and lasting declarative memory.
Trends Neurosci 21:294–299
- Cahill L, Prins B, Weber M, McGaugh JL (1994) Beta-adrenergic activation and memory for
emotional events. Nature 371:702–704
- Charles ST, Mather M, Carstensen LL (2003) Aging and emotional memory: The forgettable nature of
negative images for older adults. J Exp Psychol 132:310–324
- Christianson SA (1992a) Emotional stress and eyewitness memory: a critical-review. Psychol Bull
112:284–309
- Christianson SA (ed) (1992b) The handbook of emotion and memory: research and theory. Lawrence
Erlbaum Associates, Hillsdale
- Dolan RJ (2002) Emotion, cognition, and behavior. Science 298:1191–1194
- Dittrich WH (1994) How monkey see others: discrimination and recognition of monkey’s shape.
Behav Processes 33:139–154
- Goodall J (1986) The chimpanzees of Gombe: patterns of behaviour. Harvard University Press,
Cambridge
- Goodman GS, Ghetti S, Quas JA, Edelstein RS, Alexander KW, Redlich AD, Cordon IM, Jones DPH
(2003) A prospective study of memory for child sexual abuse: new findings relevant to the
repressed-memory controversy. Psychol Sci 14:113–118
- Hamann S (2001) Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci 5:394–400
- Heuer F, Reisberg D (1990) Vivid memories of emotional events: the accuracy of remembered
minutiae. Mem Cognit 18:496–506
- Hines D (1975) Immediate and delayed recognition of sequentially presented random shapes. J Exp
Psychol [Hum Learn] 1:634–639
- Jitsumori M, Wright AA, Shyan MR (1989) Buildup and release from proactive-interference in a
rhesus monkey. J Exp Psychol Anim Process 15:329–337
- Kendrick DF, Rilling ME, Denny MR (eds) (1986) Theories of animal memory. Lawrence Erlbaum
Associates, Hillsdale
- Kleinsmith LJ, Kaplan S (1963) Paired-associate learning as a function of arousal and
interpolated interval. J Exp Psychol 65:190–193
- Loftus EF, Burns TE (1982) Mental shock can produce retrograde-amnesia. Mem Cognit 10:318–323
- Matsuzawa T, Tomonaga M, Tanaka M (eds) (2006) Cognitive development in chimpanzees. Springer,
Tokyo
- Menzel EW (1973) Chimpanzee spatial memory organization. Science 182:943–945
- Morimura N, Matsuzawa T (2001) Memory of movies by chimpanzees (Pan troglodytes). J Comp Psychol
115:152–158
- Parr LA (2001) Cognitive and physiological markers of emotional awareness in chimpanzees (Pan
troglodytes). Anim Cogn 4:223–229
- Parr LA, Hopkins WD (2000) Brain temperature asymmetries and emotional perception in chimpanzees
(Pan troglodytes). Physiol Behav 71:363–371
- Sands SF, Wright AA (1980) Serial probe recognition performance by a rhesus-monkey and a human
with 10-item and 20-item lists. J Exp Psychol Anim Process 6:386–396
- Strange B, Hurlemann R, Dolan R (2003) An emotion-induced retrograde amnesia in humans is
amygdala- and beta-adrenergic dependent. Proc Natl Acad Sci USA 100:13626–13631
- Tanaka M (2007a) Recognition of pictorial representations by chimpanzees (Pan troglodytes). Anim
Cogn 10:169–179
- Tanaka M (2007b) Development of the visual preference of chimpanzees (Pan troglodytes) for
photographs of primates: effect of social experience. Primates 48:303–309
- Tanaka M, Tomonaga M, Matsuzawa T (2003) Finger drawing by infant chimpanzees (Pan troglodytes).
Anim Cogn 6:245–251
- Thompson RKR, Herman LM (1977) Memory for lists of sounds by bottle-nosed dolphin: convergence
of memory processes with humans. Science 195:501–503
- Tomonaga M (2007) Visual search for orientation of faces by a chimpanzee (Pan troglodytes):
face-specific upright superiority and the role of facial configural properties. Primates 6:1–12
- Tomonaga M, Tanaka M, Matsuzawa T (eds) (2003) Cognitive and behavioral development in
chimpanzees: a comparative approach. Kyoto University Press, Kyoto
- Tomonaga M, Tanaka M, Matsuzawa T, Myowa-Yamakoshi M, Kosugi D, Mizuno Y, Okamoto S, Yamaguchi
MK, Bard KA (2004) Development of social cognition in infant chimpanzees (Pan troglodytes): face
recognition, smiling, gaze, and the lack of triadic interactions. Jpn Psychol Res 46:227–235
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG (1985) Memory processing of serial lists
by pigeons, monkeys, and people. Science 229:287–289
- Zajonc RB (1980) Feeling and thinking: preferences need no inferences. Am Psychol 35:151–175



